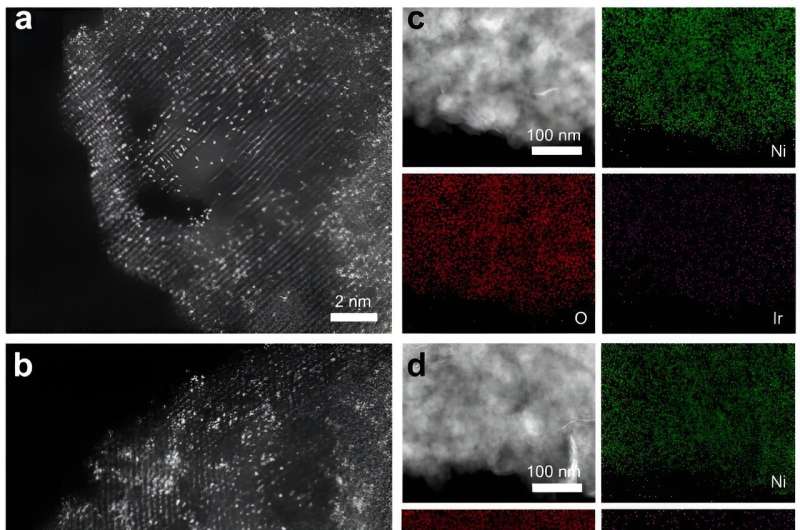
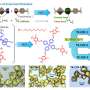
Structural characterizations of Ir1/Ni LDH-T and Ir1/Ni LDH-V. a, b HAADF-STEM images of Ir1/Ni LDH-T (a) and Ir1/Ni LDH-V (b). c, d EDS elemental mapping of Ir1/Ni LDH-T (c) and Ir1/Ni LDH-V (d). Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-44815-0
Single-atom catalysts (SACs), due to their excellent catalytic activity, have been a hot topic in the field of energy catalysis. In SACs, the metal atoms are able to directly interact with the supports, thus maximizing the metal-support interface. The metal-support interactions (MSIs) largely affect the electronic properties of single-atom catalysts and catalytic performance.
Currently, the means of regulating metal-carrier interactions are generally to replace the carrier or to treat the catalyst with hydrogen reduction, which could cause changes in the carrier or sacrifice the stability of the catalyst. Therefore, the development of a method to regulate metal-carrier interactions without changing the carriers is urgently needed.
A research team led by Prof. Zeng Jie from the Hefei National Research Center for Physical Sciences at the Microscale, the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences has constructed single-atom catalysts with efficient electrooxidation of water using site-specific MSIs. The study was published in Nature Communications.
The researchers employed an electrochemical deposition strategy to effectively modulate the site-specific metal-carrier interactions of Ir single atoms anchored on Ni layered double hydroxide (Ni LDH). Cathodic deposition drove Ir atoms anchored to triple neutral vacancies (Ir1/Ni LDH-T) and anodic deposition drove Ir atoms anchored to oxygen vacancy sites (Ir1/Ni LDH-V). Strong MSIs between Ir atoms and carriers induced the switch of active sites from Ni to Ir sites, optimizing the adsorption strength of the intermediates and thus increasing the catalytic activity.
The researchers revealed that, in accordance with the electrochemical deposition principle and X-ray absorption fine structure, Ir1/Ni LDH-T has more covalent bonds between the Ir sites and the coordinated oxygen from Ni LDH. The Ni 2p XPS peaks of Ir1/Ni LDH-T shifted to high binding energy, indicating stronger MSIs of Ir single atoms in Ir1/Ni LDH-T.
The test results of electrocatalytic water oxidation reaction showed that the mass and intrinsic activities of Ir single-atom catalysts with strong MSIs were increased by 19.5 and 5.2 times, respectively. Oxygen-isotope-labeling in situ Raman spectra showed that the 18O-labeled oxygen in Ir1/Ni LDH-V and Ni LDH was readily exchanged with the 16O atoms in the electrolyte during the water oxidation reaction, suggesting that Ni was the main active sites in these two catalysts. In contrast, 18O-labeled Ni3+–O in Ir1/Ni LDH-T would not be exchanged by 16O, indicating that Ir is the main active sites.
In addition, theoretical calculations revealed that the stronger MSI in Ir1/Ni LDH-T optimized the adsorption energy of oxygenated intermediates, thus enhancing the performance.
More information: Jie Wei et al, Site-specific metal-support interaction to switch the activity of Ir single atoms for oxygen evolution reaction, Nature Communications (2024). DOI: 10.1038/s41467-024-44815-0
Provided by Chinese Academy of Sciences