This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Harmful 'forever chemicals' removed from water with new electrocatalysis method

Scientists from the University of Rochester have developed new electrochemical approaches to clean up pollution from "forever chemicals" found in clothing, food packaging, firefighting foams, and a wide array of other products. A new Journal of Catalysis study describes nanocatalysts developed to remediate per- and polyfluoroalkyl substances known as PFAS.
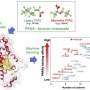
The researchers, led by assistant professor of chemical engineering Astrid Müller, focused on a specific type of PFAS called Perfluorooctane sulfonate (PFOS), which was once widely used for stain-resistant products but is now banned in much of the world for its harm to human and animal health. PFOS is still widespread and persistent in the environment despite being phased out by US manufacturers in the early 2000s, continuing to show up in water supplies.
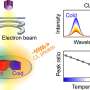
Müller and her team of materials science Ph.D. students created the nanocatalysts using her unique combination of expertise in ultrafast lasers, materials science, chemistry, and chemical engineering.
"Using pulsed laser in liquid synthesis, we can control the surface chemistry of these catalysts in ways you cannot do in traditional wet chemistry methods," says Müller. "You can control the size of the resulting nanoparticles through the light-matter interaction, basically blasting them apart."
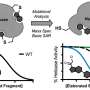
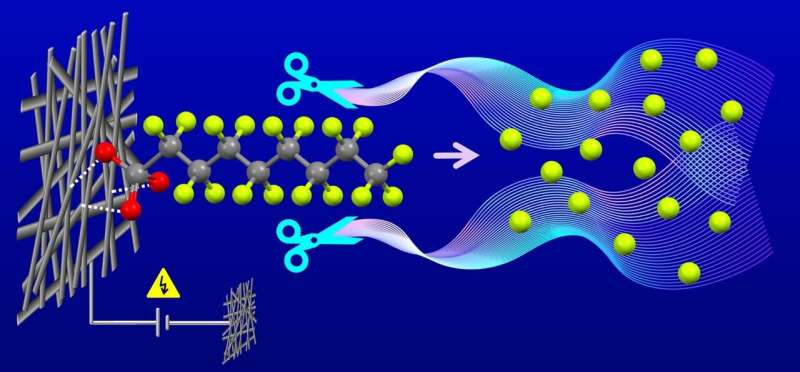
The scientists then adhere the nanoparticles to carbon paper that is hydrophilic. That provides a cheap substrate with a high surface area. Using lithium hydroxide at high concentrations, they completely defluorinated the PFOS chemicals.
Müller says that for the process to work at a large scale, they will need to treat at least a cubic meter at a time. Crucially, their novel approach uses all nonprecious metals, unlike existing methods that require boron-doped diamonds. By their calculations, treating a cubic meter of polluted water using boron-doped diamond would cost $8.5 million; the new method is nearly 100 times cheaper.
Harnessing PFAS chemicals in sustainable ways
In future studies, Müller hopes to understand why lithium hydroxide works so well and whether even less expensive, more abundant materials can be substituted to bring the cost down further. She also wants to apply the method to an array of PFAS chemicals that are still prevalently used but have been linked to health issues ranging from development in babies to kidney cancer.
Müller says that despite their issues, outright banning all PFAS chemicals and substances is not practical because of their usefulness not only in consumer products but in green technologies as well.
"I would argue that in the end, a lot of decarbonization efforts—from geothermal heat pumps to efficient refrigeration to solar cells—depend on the availability of PFAS," says Müller. "I believe it's possible to use PFAS in a circular, sustainable way if we can leverage electrocatalytic solutions to break fluorocarbon bonds and get the fluoride back out safely without putting it into the environment."
Although commercialization is a long way off, Müller filed a patent with support from URVentures and foresees it being used at wastewater treatment facilities and by companies to clean up contaminated sites where they used to produce these PFAS chemicals. She also calls it a social justice issue.
"Often in areas with lower income across the globe, there's more pollution," says Müller. "An advantage of an electrocatalytic approach is that you can use it in a distributed fashion with a small footprint using electricity from solar panels."
More information: Ziyi Meng et al, Complete electrocatalytic defluorination of perfluorooctane sulfonate in aqueous solution with nonprecious materials, Journal of Catalysis (2024). DOI: 10.1016/j.jcat.2024.115403
Provided by University of Rochester