This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Scientists deliver portable total chemical analysis without pumps and tubes
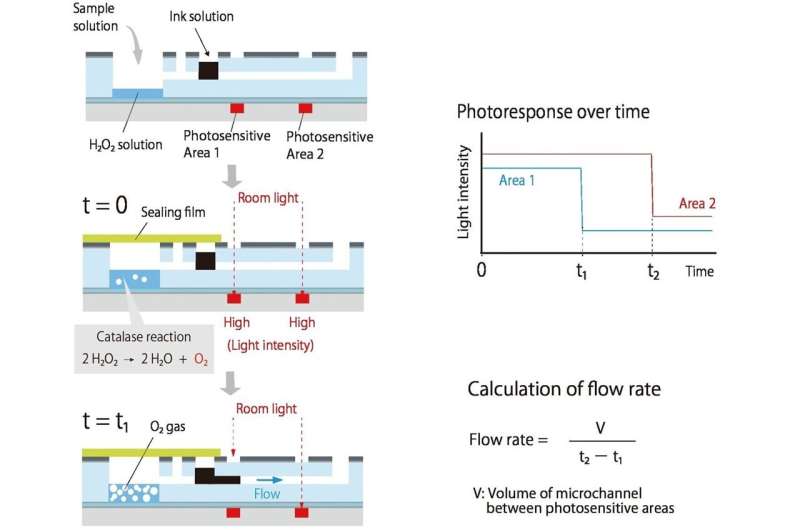
Researchers from Tokyo Metropolitan University have engineered a new micro total analysis system that quantifies a target chemical in a microfluidic chip without pumps, tubes, and expensive detectors. The compound reacts with other chemicals to produce a gas, pushing ink in a connected chamber along a channel. Built-in light detectors help measure the flow speed, allowing measurement of the original chemical. The portability of the new device enables bedside, quantitative clinical analysis.
Microfluidics is a revolutionary technology delivering precision chemistry with vastly fewer chemicals. By etching thin channels and chambers into a compact chip that can fit into the palm of your hand, chemistry can be done with microliter amounts of liquid in a vastly parallelized array of reaction conditions, saving time, cost, and the environment.
More recently, the quantitative detection of chemicals has also been incorporated into these miniature devices. These micro total analysis systems (micro-TAS) promise a complete chemical analysis that leverages all the benefits of microfluidics.
However, to drive flow around channels and chambers, microfluidics requires pumps, tubes to couple flow into channels, as well as expensive light sources and detectors to directly measure the optical signals that tell us how much of different chemicals are in our channels. This makes a method based on miniaturization and portability far less wieldy than originally proposed.
But now, a team led by Associate Professor Hizuru Nakajima from Tokyo Metropolitan University has come up with a whole new quantitation method that can get rid of the extra hardware altogether. The study is published in the journal Microchimica Acta.
They came up with a system where some compound of interest (analyte) produces a gas; the more analyte there is, the faster the gas is produced. This overpressure helps drive ink along a connected channel.
As the ink flows along, it blocks room light reaching two organic photodetectors (OPDs) printed along the channel, helping to measure the flow speed. Since the light need only be blocked by a dark ink, the detection required is inexpensive and simple. Since flow is driven by gas production, there are no pumps, and no tubes.
They demonstrated their system by measuring the amount of C-reactive protein (CRP), a protein associated with an immune system response.
Firstly, a CRP containing solution is added to a small chamber; the more CRP there is, the more attach to the specially treated walls of the chamber. Nanoparticles coated with CRP antibodies and catalase are then added; the more CRP there is, the more nanoparticles and catalase are left on the walls. When hydrogen peroxide is added, the catalase helps produce oxygen, completing the loop between analyte (in this case, CRP) and ink flow.
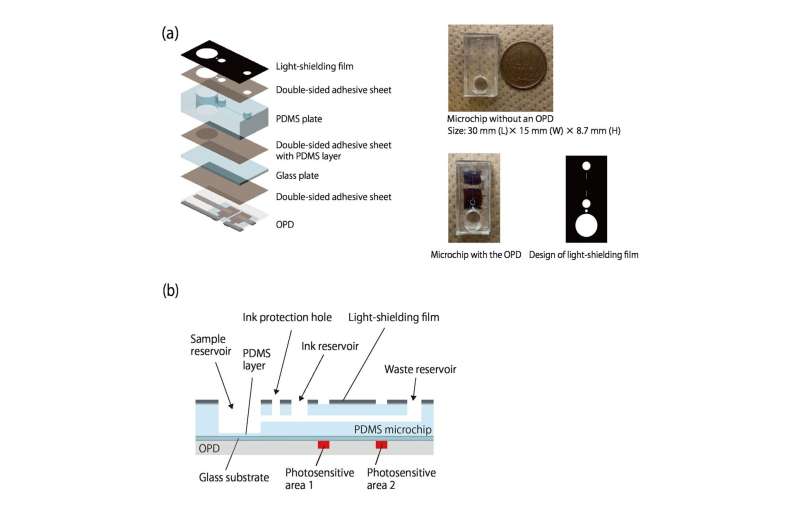
The team demonstrated that CRP concentration in human serum could be accurately detected, even in the presence of common proteins like immunoglobulin G (IgG) and human serum albumin.
There was also good agreement with commonly available, far more hardware-intensive methods. Given that the team's new chip is easily portable, they believe it will see more application of micro-TAS in clinical diagnosis by the bedside or environmental analysis in the field.
More information: Kuizhi Qu et al, Development of a C-reactive protein quantification method based on flow rate measurement of an ink solution pushed out by oxygen gas generated by catalase reaction, Microchimica Acta (2023). DOI: 10.1007/s00604-023-06108-z
Provided by Tokyo Metropolitan University