This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A 'catch-and-release' mechanism for efficient oxidation of hydrophobic aromatic organic substrates in water
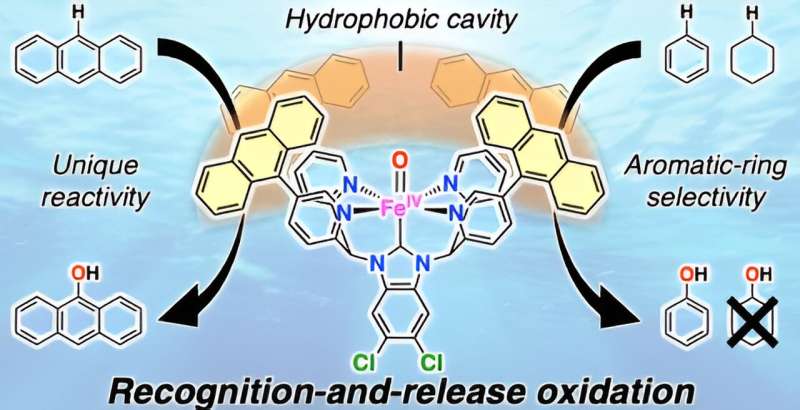
Oxidative functionalization of hydrophobic compounds is an important research area from the perspective of effective utilization of natural resources and treatment and reuse of hazardous substances. However, a method that can facilitate such reactions has not been well established.
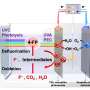
To resolve this issue, the research team at University of Tsukuba has developed a "catch-and-release" mechanism to oxidize methane to obtain methanol using an iron complex with a hydrophobic environment near the central metal as a catalyst. Using this catalyst, the team selectively and efficiently oxidized hydrophobic aromatic organic substrates in an aqueous medium under mild conditions. The study is published in ACS Catalysis.
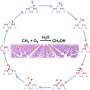
In this reaction, hydrophobic aromatic organic substrates are selectively recognized and trapped in the hydrophobic environment of iron complexes in water and hydrophilic oxidized products formed after oxidation are released into water. Based on this mechanism, they selectively oxidized hydrophobic aromatic substrates under mild conditions of 50°C in a two-phase system of aqueous solution and organic substrates using the iron complex.
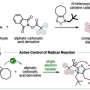
For benzene oxidation, among others, the turnover number of the involved cataytic reaction exceeded 30,000 in 3 h and 100% phenol selectivity was achieved. Furthermore, the selective two-electron oxidation of anthracene and two-electron oxidation of only aromatic compounds from mixtures of aliphatic and aromatic compounds, which has been a challenge previously, were realized.
This was achieved using a "recognition-and-release" mechanism, which represents further advancement in the catch-and-release mechanism reported previously. This recognition-and-release mechanism is expected to be an important foundation for the highly efficient and selective chemical transformation of hydrophobic aromatic organic substrates in water.
More information: Hiroto Fujisaki et al, Selective Oxidation of Hydrocarbons by Molecular Iron Catalysts Based on Molecular Recognition through π–π Interaction in Aqueous Medium, ACS Catalysis (2024). DOI: 10.1021/acscatal.3c05118
Journal information: ACS Catalysis
Provided by University of Tsukuba