This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Team expands research of water droplet interfaces that offer the secret ingredient for building life

R. Graham Cooks, the Henry B. Hass Distinguished Professor of Chemistry, and his postdoctoral researcher Lingqi Qiu have experimental evidence that the key step in protein formation can occur in droplets of pure water, and have recently published these findings in the Proceedings of the National Academy of Sciences.
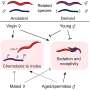
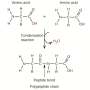
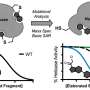
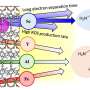
In this key step, amino acids are dehydrated (they lose water) even though they are in a water solution, a paradox that is resolved by the fact that these droplet surfaces are unusually dry and highly acidic. Under these conditions, amino acids connect to each other to create peptides, a fundamental step toward forming proteins, and eventually, living organisms.
A crucial aspect of the discovery is that the natural "left-handed" structure of amino acids is maintained throughout this process. This leads to the formation of pure chiral peptides with the same "L" handedness. The authors identified a specific compound, oxazolidinone, as a crucial intermediate in this reaction.
Further, they found that this dehydration reaction is not confined to microscopic droplets. It also happens on a larger (centimeter) scale, as demonstrated in a lab experiment starting from the oxazolidinone intermediate. This larger-scale reaction mirrors the microdroplet chemistry and is also analogous to the well-studied wet-dry cycles that are suggested to occur in hydrothermal pools and seashores. This connection links peptide formation in aerosols and in more extensive, prebiotic environments.
The study adds to the body of evidence that the surface of water drops represents a uniquely active physical and chemical system. Present are very high electric fields and extreme acidity that drive dehydration of amino acids to form peptides. Studies of the chemistry at water droplet interfaces offer new insights into the early stages of life's chemical evolution.
The authors acknowledge valuable discussions with Purdue research associates Dylan T. Holden and Nicolás M. Morato.
More information: Lingqi Qiu et al, Oxazolone mediated peptide chain extension and homochirality in aqueous microdroplets, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2309360120
Journal information: Proceedings of the National Academy of Sciences
Provided by Purdue University