This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New insight on electrochemical reactions—advancing the green transition

Electrochemical reactions are central to green transitions. These reactions use electric current and potential difference to carry out chemical reactions, which enables binding and realizing electric energy from chemical bonds. This chemistry is the basis for several applications, such as hydrogen technology, batteries, and various aspects of circular economy.
Developments and improvements in these technologies require detailed insight into the electrochemical reactions and different factors impacting them. Recent studies have shown that, besides the electrode material, the used solvent, its acidity, and the used electrolyte ions crucially impact the efficiency of electrochemical reactions.
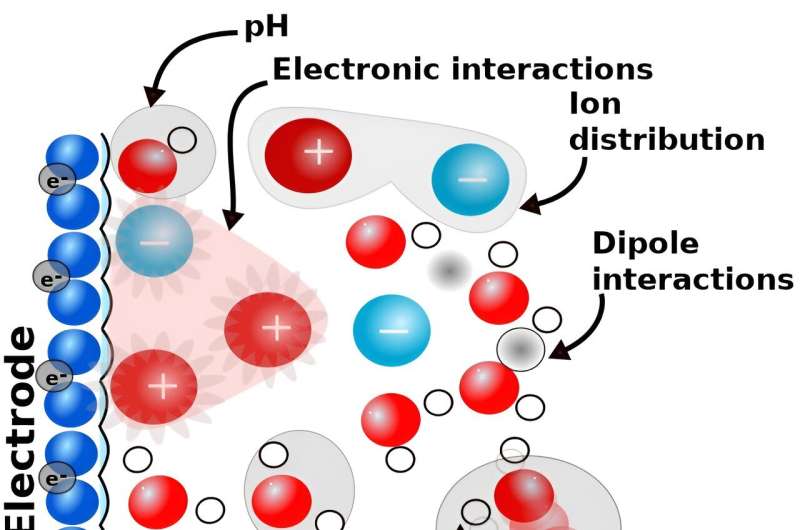
Therefore, recent focus has shifted to studying how the electrochemical interfaces, for example, the reaction environment at the electrode and the electrolyte interface impact the outcome of electrochemical reactions.
Converting carbon dioxide
Understanding the interfacial chemistry using only experimental methods is extremely difficult since they are very thin, only a fraction of a nanometer. Computational and theoretical are, therefore, crucial as they provide an accurate way to study the electrochemical interfaces at the atomic level and as a function of time.
The long-term method and theory development at the Department of Chemistry of the University of Jyväskylä (Finland) has provided a new understanding of the chemistry of electrochemical interfaces, in particular the electrolyte ion effects.
"Our two recent research articles have focused on the electrolyte ion effects in the oxygen and carbon dioxide reduction reactions, which determine the efficiency of fuel cells, hydrogen peroxide synthesis, and conversion of carbon dioxide to carbon-neutral chemical and fuels," says the Academy of Finland Research Fellow Marko Melander from Department of Chemistry of the University of Jyväskylä.
Combining experimental and computational results
Researchers at the University of Jyväskylä have collaborated with experimental and computational groups to understand the electrolyte effects. The work has been recently published in journals, Nature Communications and Angewandte Chemie International Edition.
"In both studies, we have focused on the fundamental properties and research, which has necessitated the use of highly accurate and demanding experimental and their combination with the latest simulation methods. For instance, we were able, for the first time, to combine experiments and simulations of quantum mechanical kinetic isotope effects of hydrogen to understand the oxygen reduction reaction. We also developed and applied advanced computational methods to simulate the reorganization of the aqueous electrolyte solutions to reach detailed insight on their joint effect on the reaction mechanism," elucidates Melander.
New scientific knowledge on electrochemical reactions
This research provides an atomistic picture of how electrolytes impact electrochemical reactions. One identified mechanism is the bond formation between an ion and the reacting molecule.
"We were able to show that both the ions control the structure and dynamics of both the electrode surface and the interfacial water through non-covalent interactions. These rather weak interactions then determine the reaction pathway, rate, and selectivity, and hence control the activity and outcome of electrochemical reactions," explains Melander.
Possibilities for developing renewable energy technologies
While this research focused on the fundamental aspects of electrochemical systems, it can enhance the development of improved electrochemical technologies.
"Utilizing ion and solvent effects may provide a way to tailor the reactivity and selectivity of electrochemical reactions. For instance, the electrolyte can be used to direct the oxygen reduction reaction either toward fuel cells or hydrogen peroxide synthesis applications. The electrolyte chemistry is also an effective way to steer the carbon dioxide reduction towards the wanted, valuable products," says Melander.
More information: Xueping Qin et al, Cation-induced changes in the inner- and outer-sphere mechanisms of electrocatalytic CO2 reduction, Nature Communications (2023). DOI: 10.1038/s41467-023-43300-4
Tomoaki Kumeda et al, Cations Determine the Mechanism and Selectivity of Alkaline Oxygen Reduction Reaction on Pt(111)**, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202312841
Journal information: Angewandte Chemie International Edition , Nature Communications
Provided by University of Jyväskylä