This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop chiral boryl radical catalysts in asymmetric catalysis

Researchers from the University of Science and Technology of China of the Chinese Academy of Sciences, and collaborators, have developed an innovative chiral boryl radical catalysis method, enabling asymmetric catalytic radical cycloisomerization reactions. The study was published in Science.
Chirality refers to a property of symmetry but non-superimposability. Correspondingly, each chiral compound has enantiomers, exhibiting mirror-image symmetry in spatial structure but differing in chemical properties. Asymmetric catalysis stands as a vital method for synthesizing chiral compounds.
Existing catalysts for achieving asymmetric catalysis suffer from issues such as high cost, stringent reaction conditions, and a tendency to generate pollution. However, radical catalysts demonstrate high reaction efficiency and selectivity but have a short lifespan, challenging precursor synthesis, and a relatively limited catalytic mode. Thus, achieving chiral radical-mediated asymmetric catalysis remains a challenge.
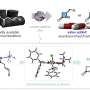
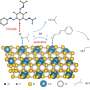
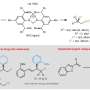
To address this challenge, the researchers designed a novel class of easily modifiable chiral N-heterocyclic carbene (NHC)-ligated boryl radicals as catalysts based on previous studies on the properties of organic boryl radicals. Leveraging the reversibility principle in the addition of unsaturated hydrocarbons, they developed a novel asymmetric cycloisomerization reaction mechanism and catalytic cycle.
These chiral boryl radical precursors, characterized by simple preparation, structural diversity, and ease of modification, laid the foundation for controlling the chirality in reactions.
The chiral boryl radicals exhibited exceptional catalytic capabilities by orchestrating four elemental steps to construct a boryl radical catalytic cycle, i.e., selective addition to alkynes, hydrogen atom transfer, intramolecular cyclization, and elimination.
During the radical cyclization step, the chiral components on NHC established a chiral environment, thereby controlling the enantioselectivity of the radical intermediates.
The researchers utilized various methods including quantum chemical calculations, electron paramagnetic resonance spectroscopy, and deuterium labeling experiments to elucidate the catalytic reaction mechanism and the source of stereoselectivity. This precision control over the catalytic process paved the way for future AI-based catalyst design by establishing a theoretical foundation.
This work not only demonstrates the potent capabilities of boryl radical catalysis in asymmetric synthesis for the first time but also serves as inspiration and impetus for the development of other main-group element radical catalysts and their asymmetric catalytic reactions.
It offers a fresh design paradigm and catalytic mode for the synthesis of chiral functional molecules.
More information: Chang-Ling Wang et al, Boryl radical catalysis enables asymmetric radical cycloisomerization reactions, Science (2023). DOI: 10.1126/science.adg1322
Journal information: Science
Provided by Chinese Academy of Sciences