This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Laser-induced hydrothermal growth for electrocatalytic applications
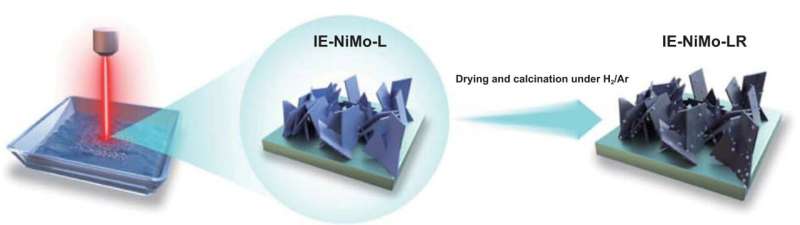
In the new study published in the journal International Journal of Extreme Manufacturing on 1 November 2023, researchers from the UK and China reported a novel technique based on a laser-induced hydrothermal reaction (LIHR) mechanism for the growth of binary metal oxide nanoarchitecture and layered-double hydroxides on nickel foams for electrocatalytic applications.
Large-scale electrochemical production of hydrogen from water splitting requires the development of electrocatalysts to overcome the kinetic energy barriers for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). The electrocatalysts need to be active, stable, and low-cost.
Among various candidates, non-precious nickel-based catalysts, particularly Ni-Mo catalysts, have gained widespread recognition for alkaline HER and layered-double hydroxides (LDHs) based on transition metals (Fe, Co, Ni) for OER catalysts in alkaline media.
However, those electrocatalysts are usually synthesized by hydrothermal or solvothermal methods, requiring autoclaves and solvents, and are also time-consuming and need high energy input.
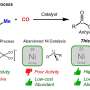
To address these challenges, the team, which pioneered the laser synthesis of electrocatalysts, further developed this alternative route to conventional hydrothermal treatment by laser irradiation of a substrate immersed in a liquid containing metal salt precursors.
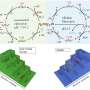
When the laser beam interaction at the interface between the liquid (containing Ni/Mo or Fe/Ni precursors) and nickel substrate generates a condition of high temperature and high pressure, which satisfies the requirement of metal oxide growth on the substrate, the growth of NiMoO4 nanosheets or NiFe-layered double hydroxide occurs on nickel foams through the hydrothermal reaction mechanism.
The first author, Dr. Yang Sha, from The University of Manchester, said, "Such nanostructures produced by the LIHR exhibit excellent catalytical activity for overall water splitting, and more importantly, with superior durability under an industrial current density, to the majority of reported catalysts, and commercial precious metal catalysts. In addition, the LIHG improves the production rate by over 19 times but only consumes 27.78% of the total energy required by conventional hydrothermal methods to achieve the same production."
Professor Zhu Liu, from the Chinese Academy of Science, Ningbo Institute of Material Technology and Engineering, commented, "LIHR was first reported in 2013 by Yeo et al. to produce local ZnO nanowires through photothermal reactions. This technique is rapid, versatile, scalable, and cost-effective, enabling direct synthesis of metal oxide nanostructures."
"However, this technique has been well understudied, and its potential applications have yet to be explored. We hope this study offers a new route for the rapid synthesis of free-standing electrocatalytic electrodes. We continue to extend its applications, including the LIHR growth of nanostructured metal oxide (ZnO, SnO2) thin-films for perovskite solar cells."
More information: Yang Sha et al, Towards a new avenue for rapid synthesis of electrocatalytic electrodes via laser-induced hydrothermal reaction for water splitting, International Journal of Extreme Manufacturing (2023). DOI: 10.1088/2631-7990/ad038f
Provided by International Journal of Extreme Manufacturing