This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
High-valence metal-doped amorphous IrOx as stable electrocatalyst for acidic oxygen evolution reaction
Hydrogen has been regarded as a potential energy carrier instead of fossil fuels, addressing energy demand and environmental issues. Proton exchange membrane water electrolysis (PEMWE), with its high energy density, elevated hydrogen purity, and rapid system response, is considered an ideal and sustainable approach to produce green hydrogen. Thus, it could be an effective solution to mitigate the intermittency and volatility of renewable energies and benefit their large-scale application.
However, the anodic oxygen evolution reaction (OER) in PEMWE involves a slow four-electron/proton transfer process, resulting in slow reaction kinetics. In addition, the localized strong oxidative and acidic environment will corrode the catalyst, leading to poor durability. Therefore, there is an urgent need to develop efficient and stable OER catalysts to boost the OER toward high-efficiency and economic hydrogen production via water splitting.
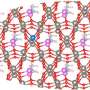
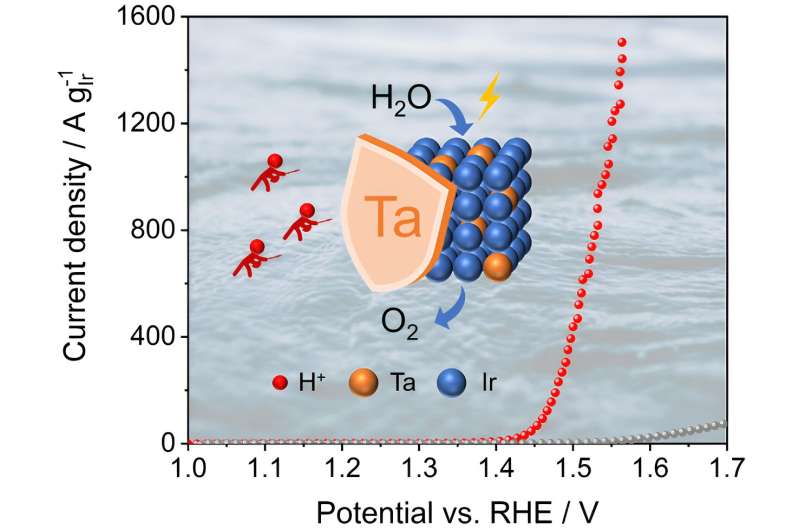
Recently, Prof. Xinbo Zhang from Changchun Institute of Applied Chemistry, Chinese Academy of Science, and collaborators have developed a series of high-valence metal-doped amorphous iridium electrocatalysts through one-step sintering for promoting the OER in acidic media. Benefiting from the modulation of geometric and electronic effects by the introduction of Ta doping and defect engineering, the enhancement of both activity and stability compared to the commercial IrO2 and IrOx was realized.
The results were published in the Chinese Journal of Catalysis.
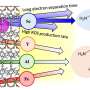
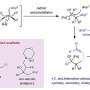
High-valent metal doping was achieved through a straightforward one-step sintering process, wherein the construction of defects was controlled by adjusting the reaction temperature. The introduction of dopants and defects is advantageous in facilitating the charge transfer, augmenting reaction sites, and enhancing the intrinsic reactivity of OER on IrOx. Simultaneously, the robust interaction of metal (Ta)-oxygen coordination further enhances the stability of IrOx during the reaction.
The optimal electrocatalyst (350-Ta@IrOx) exhibits 147.7 times higher mass activity (1207.4 A gIr–1) than that of commercial IrO2 at 1.55 V vs. RHE. Combined with the theoretical calculations, it was disclosed that Ta doping and defect engineering are conducive to the nucleophilic attack of water molecules of the rate-determining step, thereby enhancing catalytic activity and reducing the overpotential of OER on IrOx.
In addition, it demonstrates no noticeable performance degradation during the 500 h durability test, obviously outperforming the undoped sample and commercial IrO2 and claiming its potential for industrial applications.
More information: Ning Zhang et al, High-valence metal-doped amorphous IrO as active and stable electrocatalyst for acidic oxygen evolution reaction, Chinese Journal of Catalysis (2023). DOI: 10.1016/S1872-2067(23)64517-6
Provided by Chinese Academy of Sciences