This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Synthetic biology tool comprehensively reveals gene regulatory networks in E. coli
The intricate interplay of gene expression within living cells is akin to a well-orchestrated symphony, with each gene playing its part in perfect harmony to ensure cells function as they should. At the heart of this symphony are transcription factors (TFs), molecular maestros that regulate the expression of genes by binding to specific DNA sequences known as promoters.
Unlocking the secrets of these genome-scale regulatory networks requires a comprehensive collection of gene expression profiles, but measuring gene expression responses for every TF and promoter pair has posed a formidable challenge due to the sheer number of potential combinations, even in relatively simple organisms such as bacteria.
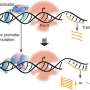
To tackle this challenge, researchers led by Fuzhong Zhang, professor of energy, environmental & chemical engineering in the McKelvey School of Engineering at Washington University in St. Louis, developed a technique called pooled promoter responses to TF perturbation sequencing (PPTP-seq).
PPTP-seq integrates CRISPR gene editing with a combinatorial library containing every known TF in the target genome and corresponding promoters. The groundbreaking technique allows scientists to examine gene regulation by hundreds of TFs acting on thousands of promoters in a single experiment, which takes about two weeks to complete and produces easily processable data.
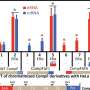
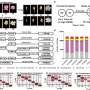
In a study published online Sept. 16 in Nature Communications, first author Yichao Han, who earned a doctorate in environmental engineering in 2023 while working in Zhang's lab, used PPTP-seq to systematically explore the activity of 1,372 E. coli promoters when subjected to perturbation of each of the 183 TFs.
This single experiment provided insight into more than 200,000 potential TF-promoter interactions, painting a comprehensive picture of E. coli's regulatory landscape, revealing novel regulatory responses and setting the stage for future genetic exploration.
"As a synthetic biology tool, PPTP-seq allows high-throughput study of gene regulation," Zhang said. "The most commonly used tool to study gene regulation, RNA-seq, can reveal around 4,000 regulator-gene responses. PPTP-seq increased this number by 50-fold, revealing 200,000 regulator-gene responses from a single experiment. Using this powerful new tool, we were able to provide a comprehensive regulatory network for E. coli at the genome-scale."
In addition to providing a lot more data, PPTP-seq also can capture the intricate nuances of gene regulation in different cellular contexts. By subjecting E. coli to varying growth media, the researchers uncovered complex promoter activities and gene regulation, offering a glimpse into the adaptability of these microorganisms.
"Even simple bacteria have more than 4,000 genes," said Han, who is now a researcher at Pacific Northwest National Laboratory. "TFs control gene expression levels, turning genes off and on in response to environmental stimuli. However, these controls aren't always direct. The downstream effects of activating one TF could involve multiple TFs and genes."
Han observed that gene regulation is not a one-size-fits-all phenomenon, but rather a finely tuned process dependent on environmental cues. Because the gene regulation network is complex, previous tools to study it have proven onerous in terms of time and effort required. With its ability to precisely perturb individual TFs using CRISPR and record downstream effects for all TF and promoter combinations, PPTP-seq reveals how different promoters respond under different conditions, underscoring the dynamic nature of gene expression, even in simple organisms.
"Ultimately, PPTP-seq allows us to perform systems biology studies with much greater efficiency—50-fold higher than current approaches," Zhang said. "Our work has revealed a comprehensive regulatory response network in bacteria, with many previously unknown responses."
This deeper understanding of cellular regulation could have far-reaching implications in fields such as biotechnology and medicine. Han specifically pointed to potential applications in biomanufacturing, where newly discovered downstream responses could be harnessed to program bacteria to produce desired materials, even under non-ideal conditions. Beyond bacteria, PPTP-seq could eventually be used to study more complex cells, including animal cells.
"A big advantage of the PPTP-seq technology is that we're studying the entire gene regulation network all in one go, uniformly checking for all interactions at various conditions, so we can make direct comparisons," Han said. "Right now, this is basic science. We're looking at E. coli as a model organism because it's already well studied, but we still discovered new things about it. That suggests that there will be even more to learn as we expand into other cells and applications."
More information: Yichao Han et al, Genome-wide promoter responses to CRISPR perturbations of regulators reveal regulatory networks in Escherichia coli, Nature Communications (2023). DOI: 10.1038/s41467-023-41572-4
Journal information: Nature Communications
Provided by Washington University in St. Louis