This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers challenge long-standing theory guiding nanoparticle treatment of tumors
Researchers at the University of Toronto have developed a new theory to explain how nanoparticles enter and exit the tumors they are meant to treat, potentially rewriting an understanding of cancer nanomedicine that has guided research for nearly four decades.
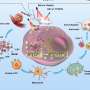
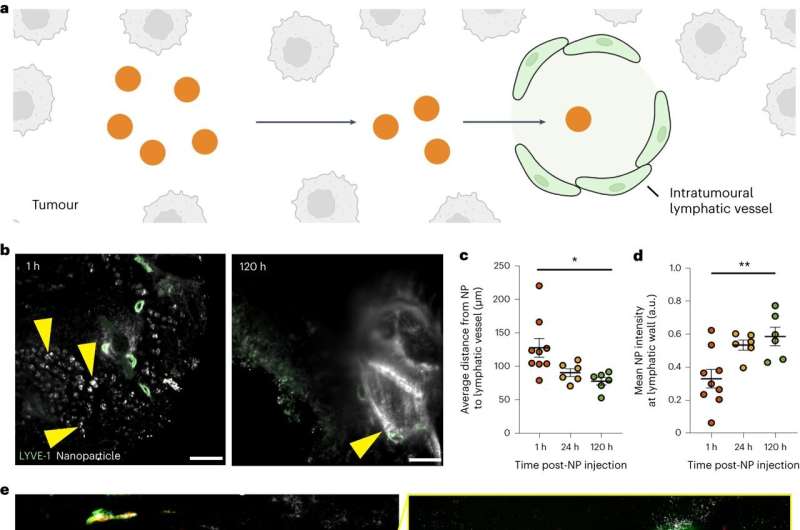
The Enhanced Permeability and Retention (EPR) effect, a concept largely unchallenged since the mid-1980s, posits that nanoparticles enter a tumor from the bloodstream through gaps between the endothelial cells that line its blood vessels—and then become trapped in the tumor due to dysfunctional lymphatic vessels.
"The retention aspect of the EPR theory is contingent on the lymphatic vessel cavity being too small for nanoparticles to exit, thereby helping nanoparticles that carry cancer-fighting drugs to stay in the tumors," said Matthew Nguyen, a Ph.D. student in the Institute of Biomedical Engineering in the Faculty of Applied Science & Engineering and the Donnelly Center for Cellular and Biomolecular Research,
"But we found around 45 percent of nanoparticles that accumulate in tumors will end up exiting them."
Nguyen, who is a member of the lab of Professor Warren Chan, is the lead author on a new study recently published in the journal Nature Materials that challenges the long-standing theory. The researchers' findings help explain why treatments based on the EPR effect are failing in clinical trials, building on earlier research from the Chan lab that showed less than one percent of nanoparticles actually reach tumors.
The researchers found that, contrary to the EPR effect, nanoparticles can leave tumors through their lymphatic vessels. The exit method for a nanoparticle depends on its size, with larger ones (50–100 nanometers wide) more likely to leave through lymphatic vessels in the tumors, and smaller ones (up to 15 nanometers wide) more likely to leave through lymphatic vessels surrounding the tumors.
In rare cases, nanoparticles will exit through blood vessels.
Nanoparticle exit from tumors occurs through spaces in the lymphatic vessel walls and transport vesicles that carry them across these walls. The researchers showed that nanoparticles will re-enter the bloodstream following lymphatic drainage, and hypothesized that these nanoparticles will eventually return to the tumor for another opportunity to treat it.
Disproving the EPR effect was no easy feat. The Chan lab spent six years working to understand why nanoparticles do not accumulate in tumors effectively. Prior to this study, the lab focused on how nanoparticles enter tumors in the first place. Through this and other studies, the lab developed a competing theory to the EPR effect, called the Active Transport and Retention (ATR) principle.
Nguyen noted that the field of nanomedicine has evolved since the publication of the nanoparticle entry study in 2020. "We got more pushback from other researchers on that study compared to this one," he said. "People have started to accept that the EPR effect is flawed."
With nearly half of accumulated nanoparticles exiting tumors, mostly through lymphatic vessels, future research could address this issue through nanoparticle treatments that prevent lymphatic drainage.
"We are excited to have a better understanding of the nanoparticle tumor delivery process," said Chan. "The results of these fundamental studies on nanoparticle entry and exit will be important for engineering nanoparticles to treat cancer."
The study's findings, if applied across the field of cancer nanomedicine, promise a new direction to improve our understanding of how nanoparticles can be used to treat tumors.
"Trying to translate cancer nanomedicine to the clinic is like a working with a black box—some drugs work, some don't, and it's difficult to know why," said Gang Zheng, associate research director at the Princess Margaret Cancer Center and a professor of medical biophysics in U of T's Temerty Faculty of Medicine who was not involved in the study.
"Chan's dedication to better understanding the mechanisms of nanoparticle uptake and exit is shining light on these processes to help make our translation efforts more efficient and successful."
More information: Luan N. M. Nguyen et al, The exit of nanoparticles from solid tumours, Nature Materials (2023). DOI: 10.1038/s41563-023-01630-0
Journal information: Nature Materials
Provided by University of Toronto