This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Discovery in mosquitoes could lead to new strategy against dengue fever and other mosquito-borne vectors
Researchers from the Johns Hopkins Malaria Research Institute at the Johns Hopkins Bloomberg School of Public Health have made an important finding about Aedes aegypti mosquitoes—one that could one day lead to better methods for reducing the mosquito-to-human transmission of dengue, yellow fever, Zika, and other harmful and sometimes deadly viruses.
Ae. aegypti mosquitoes do not succumb to these viruses when infected and continue to move and feed normally. As such, the infected mosquitoes can pass their viral cargoes on to humans. The researchers discovered that an Ae. aegypti protein, Argonaute 2, has a key role—via several biological mechanisms—in keeping mosquitoes healthy and active despite these infections.
The discovery represents a significant advance in understanding mosquito biology. It also hints at a strategy that would aim to shut down Ae. aegypti mosquitoes' defenses whenever they become infected by certain viruses—killing the mosquitoes and thereby reducing the transmission of those viruses by Ae. aegypti to humans.
Instead of making mosquitoes more resistant to the viruses, the discovery opens a possible path for making mosquitoes more susceptible and less tolerant to virus infection, which would impair their ability to transmit disease.
The research was published online September 18 in Nature Communications.
"Researchers have long wondered why Ae. aegypti mosquitoes don't get sick when they are infected by these viruses—our findings effectively solve this mystery and suggest a potential new mosquito-based disease control strategy that merits further study," says study senior author George Dimopoulos, Ph.D., a professor in the Johns Hopkins Malaria Research Institute and in the Bloomberg School's Department of Molecular Microbiology and Immunology.
The study's lead author was Shengzhang Dong, Ph.D., a senior research associate in the Bloomberg School's Department of Molecular Microbiology and Immunology.
Ae. aegypti mosquitoes transmit "arthropod-borne" or "arbo-" viruses including dengue virus, yellow fever virus, Zika virus, chikungunya virus, and Mayaro virus. Each year these pathogens sicken millions of people around the world each year, killing tens of thousands. There are no antiviral therapies for any of these viruses.
Currently, a vaccine is available for yellow fever virus. One dengue vaccine is approved by the Food and Drug Administration for individuals between six and 16 who have had prior dengue infection. Disease control methods for Ae. aegyptiemphasize the use of insecticides, which have had limited success and have led to insecticide resistance.
Ae. aegypti mosquitoes are effective vectors of arborviruses because they can sustain significant infections with these viruses without suffering costs to their overall ability to reproduce—what biologists call "fitness." If the mosquitoes' fitness was impaired, they would likely have evolved strong defenses against these pathogens. Instead, they somehow ended up with a live-and-let-live balance that allows them to carry at least moderate viral loads without apparent adverse effects.
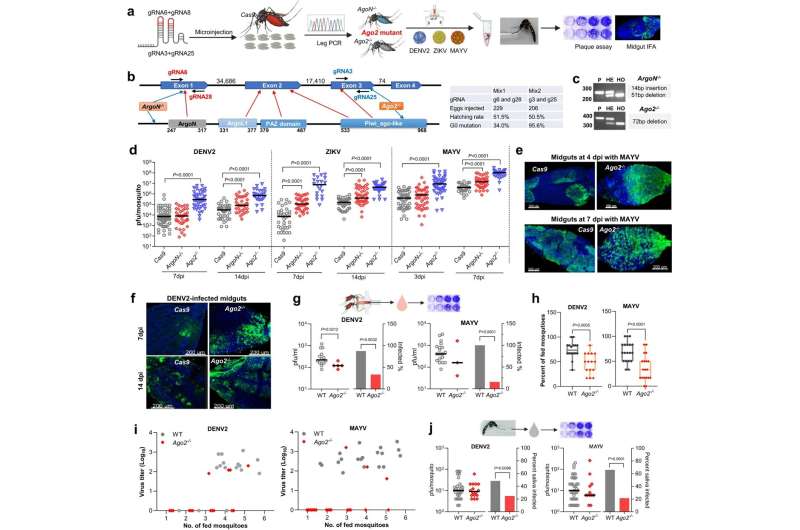
In the new study, Dimopoulos and Dong examined the role of Argonaute 2 (Ago2), a protein that in mosquitoes serves as part of an important antiviral mechanism known as the small interfering RNA (siRNA) pathway, which works by recognizing and destroying viral RNAs.
The researchers found that in Ae. aegypti mosquitoes lacking the Ago2 gene, the siRNA pathway is impaired, arborvirus infection becomes more severe, and the mosquitoes' ability to transmit these viruses drops sharply—as they sicken, feed less, and often die within days.
The scientists showed that this increased mortality is caused not only by the impairment of the siRNA antiviral pathway, but also by defects in two other processes that happen to depend on Ago2: DNA repair, and a basic waste-removal process called autophagy. Ago2-deficient mosquitoes exposed to arborviruses were left with hyperinfections, extensive DNA damage, and the accumulation of molecular waste in their dying cells.
Apart from illuminating an important aspect of Ae. aegypti biology, the findings point to a possible new arboviral disease control strategy. This would be to engineer the mosquitoes so that arbovirus infections trigger the loss of their tolerance mechanisms, perhaps via the inhibition of Ago2. Arborvirus-carrying Ae. aegypti mosquitoes would thus die quickly, whereas the much greater number of non-arborvirus carrying Ae. aegypti should be unaffected.
"The biology of mosquito susceptibility and tolerance to infection is an interesting area of exploration for other pathogens as well," says Dimopoulos. "For instance, mosquitoes that transmit malaria parasites could perhaps also be engineered to become sick and succumb to infection."
Dimopoulos and his research group are now exploring possible ways of engineering Ae. aegypti to test this possible new disease-control strategy.
More information: Shengzhang Dong et al, Aedes aegypti Argonaute 2 controls arbovirus infection and host mortality, Nature Communications (2023). DOI: 10.1038/s41467-023-41370-y
Journal information: Nature Communications