This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Researchers use carbon capture and utilization technology to recycle industrial carbon dioxide
Climate change is a serious concern that needs to be prioritized globally. Nations across the globe are drafting policies to reduce the impact of global warming and climate change. For instance, the European Union has recommended a comprehensive set of guidelines to achieve climate neutrality by 2050. Likewise, the European Green Deal puts heavy emphasis on reducing greenhouse gas emissions.
The capture of emitted carbon dioxide (CO2) and its chemical conversion into useful commercial products is one way to limit global warming and mitigate its effects. Scientists are now looking into carbon capture and utilization (CCU) technology as a promising approach to expanding CO2 storage and conversion at a low cost.
Global CCU research, however, is largely limited to only about 20 conversion compounds. Given the variety of CO2 emission sources, it is critical to have a wider range of chemical compounds, which necessitates delving deeper into processes that can convert CO2 even at low concentrations.
A team of researchers from Chung-Ang University in Korea are conducting research on CCU processes that use waste materials or abundant natural resources as raw materials to ensure their economic feasibility.
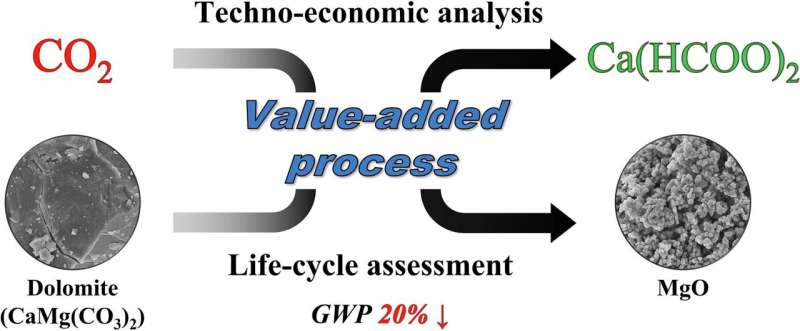
The team, led by Professor Sungho Yoon and Associate Professor Chul-Jin Lee, recently published a study where they discuss the utilization of industrial CO2 and dolomite—a common and abundant sedimentary rock that is a rich source of calcium and magnesium—for the production of two commercially viable products: calcium formate and magnesium oxide.
The study was published in the Chemical Engineering Journal.
"There is a growing interest in utilizing CO2 to produce valuable products that can help mitigate climate change while creating economic benefits. By combining CO2 hydrogenation and cation exchange reaction, a process for simultaneous metal oxide purification and high-value formate production has been developed," remarks Prof. Yoon.
In their study, the researchers used a catalyst (Ru/bpyTN-30-CTF) to add hydrogen to CO2, which resulted in the production of two value-added products, calcium formate and magnesium oxide. Calcium formate, a cement additive, de-icing agent, and animal feed additive, is also used in leather tanning.
Magnesium oxide, in contrast, is extensively used in the construction and pharmaceutical industries. The process was not only viable but also extremely rapid, yielding the products in just 5 minutes at room temperature. Moreover, the researchers estimated that this process could reduce global warming potential by 20% when compared to traditional calcium formate production methods.
The team also evaluated if their method could potentially replace the current production approaches by checking its environmental impact and economic feasibility. "Based on the results, we can say that our method offers an eco-friendly CO2 conversion alternative that could replace the conventional approaches, potentially contributing to the reduction of industrial CO2 emissions," Prof. Yoon explains.
Although converting CO2 into meaningful products sounds promising, these processes are not always easy to scale up. Most of the CCU technologies have not been commercialized owing to their low economic feasibility compared to the prevailing commercial processes. "We need to combine CCU processes with waste material recycling to make them both environmentally and economically beneficial. This may contribute to achieving a net-zero emissions goal in the future," concludes Dr. Lee.
More information: Hayoung Yoon et al, Kinetic conversion of magnesium and calcium ions of dolomite into useful value-added products using CO2, Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.143684
Provided by Chung Ang University