This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
'Spider-like' mitochondrial structure initiates cell-wide stress response
Often referred to as the "powerhouses of the cell," mitochondria are well known for their role as energy suppliers, but these organelles are also critical for maintaining our overall health. Mitochondrial stress is associated with aging and age-related diseases, including neurodegeneration, but there has been a limited understanding of the molecular mechanisms behind this mitochondrial stress signaling. Now, a study by Scripps Research scientists has revealed an important step in this process.
The new study, published August 7, 2023, in the journal Nature Structural & Molecular Biology, shows how a mitochondrial protein structure is necessary to activate the cell's integrated stress response (ISR)—a critical pathway that helps our cells maintain health. The researchers believe this mitochondrial structure, made up of a protein called DELE1, could serve as a target for future therapeutics for age-related diseases.
"Understanding the molecular details of this signaling pathway could help us potentially develop treatments for a range of diseases, such as neurodegenerative diseases, cancer and heart disease," says first author Jie Yang, Ph.D., a postdoctoral fellow in the lab of Gabriel Lander at Scripps Research.
In order to maintain cellular function and health, mitochondria must continually sense and respond to stressors, such as viral infections and iron deficiency. However, their ability to do so decreases as people age.
"Just like every other part of our body, mitochondria age and become slightly less productive," says co-author Kelsey Baron, a graduate student in the lab of Luke Wiseman at Scripps Research. "When you have this loss of mitochondrial productivity, your cells don't have as much energy to fight different stressors, and many people believe that is a major trigger of neurodegeneration."
One method by which mitochondria deal with stress is by activating the ISR. Prior studies have shown that the DELE1 protein is involved in activating this integrated stress response, but before now, little was known about the protein's molecular structure. Characterizing DELE1's structure is a key step towards understanding and treating diseases associated with mitochondrial stress.
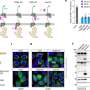
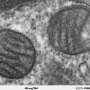
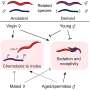
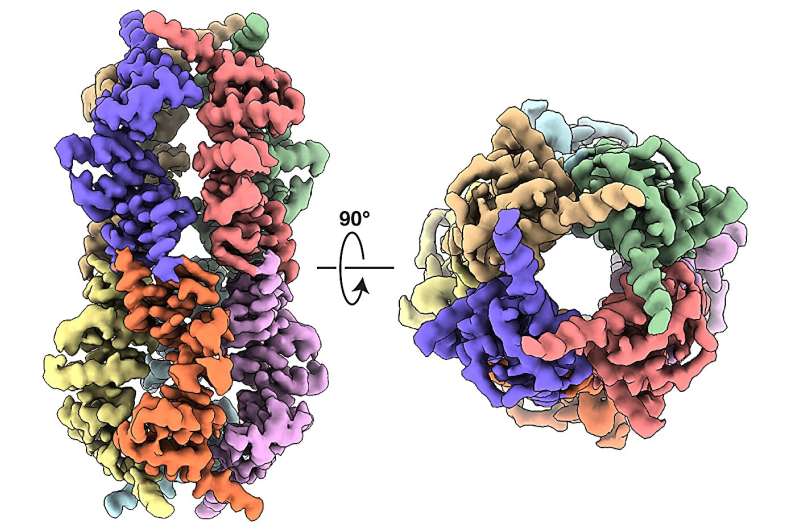
The researchers focused on a fragment of DELE1—the C terminus—that is known to be actively involved in initiating the ISR. When they isolated this fragment, they were surprised to find that it was much heavier than expected, which suggested that multiple copies of the protein fragment were binding together. Using electron microscopy, the team showed that this protein complex (or oligomer) was a highly symmetrical cylinder composed of eight identical fragments—in other words, an octamer.
"It was completely unexpected that it was forming this much larger, oligomeric structure," says study co-senior author Gabriel Lander, Ph.D., professor in the Department of Integrative Structural and Computational Biology at Scripps Research. "It's kind of like two four-legged spiders whose legs are intertwined to form this flexible cylindrical structure."
The researchers captured more than 12,000 electron microscope images of the octamer and then used algorithms to produce a three-dimensional structural model. Then, by looking at the positions of different amino acids (the building blocks of proteins) within the structure, they were able to identify which amino acids are involved in binding and assembling the octamer.
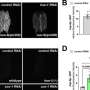
To test whether this oligomerization of DELE1 is required to activate the ISR, the researchers then introduced mutations into some of the key amino acids, which would disrupt the ability of DELE1 to bind together. When they cultured cells that contained this mutated, un-oligomerizable version of DELE1, the cells were unable to activate the ISR—suggesting that oligomerization is critical to activating this stress signaling pathway.
The next step is to find ways to use this structural information to manipulate these pathways—notably in different diseases and disorders, the researchers say.
"Knowing that this oligomerization step is a potential site of regulation gives us a platform for potential drug development," says co-senior author Luke Wiseman, Ph.D., professor in the Department of Molecular medicine at Scripps Research. "We think that targeting this pathway has potential for improving outcomes in a variety of different disorders."
As well as Jie Yang, Kelsey Baron, Luke Wiseman, and Gabriel Lander, authors of the study "DELE1 oligomerization promotes integrated stress response activation," include Daniel E. Pride, Anette Schneemann, Wenqian Chen, and Albert S. Song of Scripps Research; and Xiaoyan Guo, Giovanni Aviles and Martin Kampmann of the University of California, San Francisco.
More information: Jie Yang et al, DELE1 oligomerization promotes integrated stress response activation, Nature Structural & Molecular Biology (2023). DOI: 10.1038/s41594-023-01061-0
Journal information: Nature Structural & Molecular Biology
Provided by The Scripps Research Institute