This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unlocking the mysteries of freezing in supercooled water droplets
Clouds are a lot cooler than you might think. In fact, scientists might say they're super cool because they're made up of millions of supercooled water droplets, droplets that have been cooled below the freezing point but haven't yet turned into ice. When these droplets freeze, they can accelerate freezing of the whole cloud through a process called secondary ice production. This is a rapid, complex process that happens across different time and length scales.
"Researchers in the atmospheric science community are trying to understand how ice is being produced so efficiently in clouds, and also what type of ice forms," said Claudiu Stan, a scientist at Rutgers University. "When ice forms in supercooled water, the water is going to freeze much faster than if you were waiting for ice to form in a freezer. And what people have seen before is you don't get the same kind of ice that you get from the freezer. But until now, it's been quite difficult to see what is happening at the very beginning of freezing."
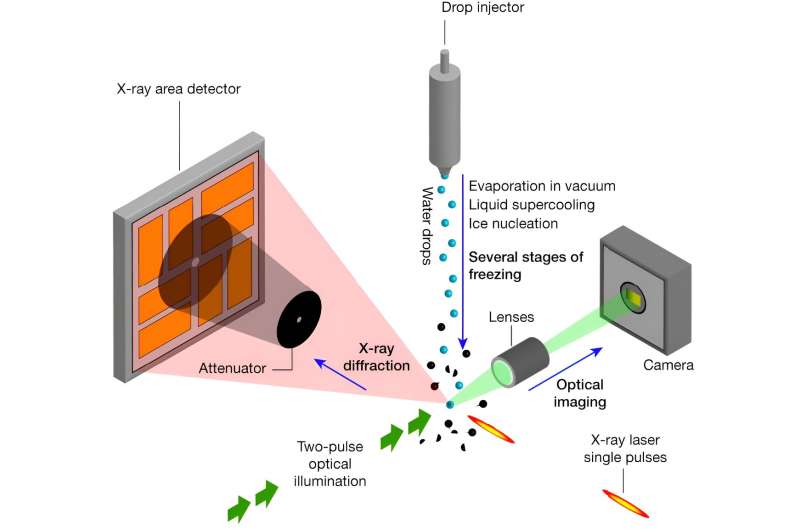
A team of researchers has been exploring this intricate process more closely using the Linac Coherent Light Source (LCLS) X-ray laser, located at the Department of Energy's SLAC National Accelerator Laboratory.
The researchers developed a model of the freezing process that includes seven distinct stages and discovered an unexpected structure along the way. Their results, published today in Nature, could improve our understanding of cloud behavior and its effect on the climate.
"The freezing of these tiny droplets is a phenomenon that isn't fully understood, and it might contain clues that could help us better understand climate change," said Stan, who led the study. "We discovered that the process is actually more complicated than one might think."
Unexpected structure
At LCLS, the team used two techniques to study tens of thousands of water microdroplets: optical microscopy, which magnifies small objects by bending light through a series of lenses, and X-ray diffraction, a technique where scientists hit a sample with X-rays and look at the pattern they make when they bounce off to figure out how its atoms or molecules are arranged.
They realized that the earliest steps of freezing are almost independent of the environmental factors, so they cooled the drops rapidly in a vacuum to help capture these steps. Diffraction from the formed ice crystals revealed that a crystalline arrangement formed shortly after the freezing process started.
Diffraction from the remaining liquid between ice crystals showed patterns similar to what one might see on the surface of ice, where there's a super-thin layer that isn't quite liquid or solid. The researchers also found that the ice forms a strained, or stressed, hexagonal crystal arrangement right after freezing. This unexpected structure, which had not been observed before, is a temporary, unstable state that is likely to precede the formation of ice with other kinds of abnormalities in the crystal structure.
"This freezing happens extremely quickly and in a very small region," said SLAC scientist Sebastien Boutet. "This is where the X-ray laser comes into play. It allows us to follow these ultrasmall, ultrafast changes, taking snapshots of the molecules in the crystal to see how they behave throughout the process."
Turning into ice
The freezing of the supercooled drops goes through different stages. The researchers identified seven of these stages in their system and arranged them into a predictive model. First, a tiny piece of ice nucleates in the super cold water. Then ice starts to grow in tree-like patterns, making the droplet change shape a bit and freezing half the water inside.
Next, a smooth outer ice layer forms around a middle part that has both the tree-like ice patterns and some liquid. Since water expands as it turns to ice, small, pointy ice structures start to show up on the droplet's surface as the inside liquid is pushed out through cracks in the outer ice layer.
Soon, the droplet forms more and larger pointy ice structures. The expanding ice causes some droplets to crack but not break apart. Some other droplets completely break into pieces because of the pressure from the expanding ice.
"This a quite complicated process, because some of these stages start at variable times and places, so each drop freezes in a slightly different way," Stan said. "At first we were not sure that it could be modeled with the data we had, but we were able to make a model that has seven freezing stages and also predicts a slightly different freezing for each drop. Because of our success, we think that such freezing models can also be made for droplets in clouds."
Completing the picture
Now that they have a better idea of what happens in the beginning of this freezing process, the researchers plan to conduct follow-up experiments at different points in time to get a more complete picture.
The research provides a detailed understanding of how supercooled water droplets freeze, which could lead to new insights into atmospheric processes and the wider climate system. Beyond this, the techniques used could help researchers better understand the freezing or solidification process in other materials or under meteorological conditions.
"When we started this experiment, we thought that we would confirm a metastable structure of ice crystals previously seen at longer time delays, and we ended up observing something that was quite unexpected," Stan said. "Now we have a better understanding of what's happening at the beginning of freezing, which I find quite exciting."
More information: Armin Kalita et al, Microstructure and crystal order during freezing of supercooled water drops, Nature (2023). DOI: 10.1038/s41586-023-06283-2
Journal information: Nature
Provided by SLAC National Accelerator Laboratory