This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unraveling the super-complex structure of supercooled liquids
When cooled to their freezing point, most liquids become solids or crystallize. In other words, the molecules arrange themselves in a perfectly ordered fashion, which physicists call a crystal. Supercooled liquids are different; they do not form such crystals even if they are cooled below their freezing point. These liquids are used in many industries, but a thorough understanding of their properties is lacking. Eindhoven University of Technology (TU/e) researchers now reveal the most realistic description of their properties to date, using—as a first-time—four body correlation functions. The work is published in the journal PNAS Nexus.
Take a look around you. What do you see? Perhaps you see books, papers, and magazines? Or if you're outside, you see trees, plants, and fields as far as the eye can see. If whatever you see is neatly arranged, then this order can be pleasing to the eye, but once the objects become muddled or disordered, to many this can be unpleasant and messy.
In the macroworld, it's easy to tell if a system is neat or messy, ordered or disordered. Zooming into the scale of atoms and molecules in substances is another challenge though. While solids typically have an ordered crystalline structure, the same can't be said for liquids.
Normal liquids versus supercooled liquids
For a normal liquid with the temperature is above its freezing point but below its boiling point, its molecules are constantly moving, and they are free to explore the volume confining them. In other words, the molecules do not have well-defined positions relative to each other.
However, once the liquid is cooled to its freezing point, a phase transition occurs with the liquid molecules arranging themselves in a very specific way to form a crystalline solid.
Water is an everyday example where molecules move freely above zero degrees Celsius, but are locked into a crystalline solid phase (ice) when the temperature is below zero degrees Celsius. However, the situation with supercooled liquids is quite different.
"A supercooled liquid is one that can be cooled down or compressed below its freezing point without turning into a crystal," says Ilian Pihlajamaa, Ph.D., researcher in the Soft Matter & Biological Physics group at TU/e.
So, why does this happen? Corentin Laudicina, Ph.D., a colleague of Pihlajamaa at TU/e explains, "In some cases, if the cooling is very fast, the liquid can remain in a liquid state even if the temperature is below the normal freezing point. As a result, it has some unique properties that can be useful for a variety of industrial applications, from sustainable plastics to high-tech optical materials."
Moving beyond pairs
With supercooled liquids having such a wide range of applications, it would be beneficial to have a better understanding of how the molecules in supercooled liquids influence each other, as these interactions can significantly affect overall liquid properties.
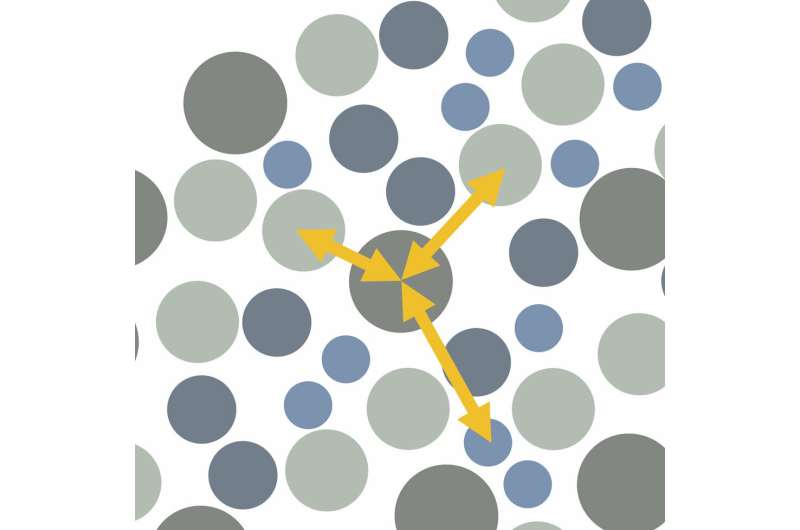
One of the most popular tools to describe a liquid's structure is a two-molecule or two-body structural correlation, which examines how any two molecules affect or correlate with each other. As would be expected by intuition, molecules that are closer tend to have greater influence on a molecule of interest than molecules that are further away from the molecule of interest.
"Two-molecule correlations are useful, but we would like to know more about the influence of multiple molecules on a given molecule at the same time," says Pihlajamaa. "At the fundamental level, to describe a liquid's structure in more detail, multiple-molecule correlations are needed. Moving beyond two- and three-molecule correlations provides these details."
In the paper published in PNAS Nexus, joint first authors Pihlajamaa and Laudicina, along with their supervisor Liesbeth Janssen from TU/e and Chengjie Luo from the Max Planck Institute for Dynamics and Self-Organization in Göttingen, have moved beyond conventional pair correlations by calculating many-body correlations using simulations and new theory.
Calculation challenges
"Most studies to date focus only on two-body structural correlations, and just a handful of papers have sought to consider additional three-body correlations. We have gone further than anyone else in terms of the calculations by being the first to look at four-body correlations," says Janssen.
"We know supercooled liquids have different properties to normal liquids. The laws of physics indicate these properties are connected to the liquid's molecular structure," says Laudicina. "There's little difference between two- and three-molecule correlations for supercooled liquids, but the four-molecule correlations reveal locally preferred structures not seen before."
The work presented the researchers with several challenges. "The mathematical derivations were fairly long and technical," says Laudicina. "We had to deal with tens to hundreds of terms in the equations, so verifying whether or not this was correct presented an interesting challenge," adds Pihlajamaa.
Added to that, to measure four-body correlation functions, the researchers needed data, and lots of it. "We used advanced computer programs on high-performance clusters to run our in-house developed code. Our laptops were definitely not capable of running this code," says Pihlajamaa.
Twenty years and counting
For the researchers, this was a significant breakthrough, particularly when combined, the four have more than twenty years' experience working on better theories to explain correlations between molecules in liquids.
"Breakthroughs like this will inspire me to continue to work on these novel ideas further and to improve upon the best theories out there," says Janssen.
"Contributing to the understanding of the complex behavior of dense liquids and pushing the boundaries of what is currently known brings a sense of fulfillment and satisfaction," adds Laudicina.
The deeper insight on the structure of supercooled liquids could pave the way for new materials for application in materials engineering, manufacturing processes, chemical engineering, and the design of energy storage systems.
"It's all about improving the performance, efficiency, and sustainability of new materials. But to do this, it is important to improve our understanding of the fundamental aspects of these systems, and our research is a major stepping stone in the right direction," notes Janssen.
More information: Ilian Pihlajamaa et al, Emergent structural correlations in dense liquids, PNAS Nexus (2023). DOI: 10.1093/pnasnexus/pgad184
Journal information: PNAS Nexus
Provided by Eindhoven University of Technology