This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Study uncovers the secrets of plant regeneration
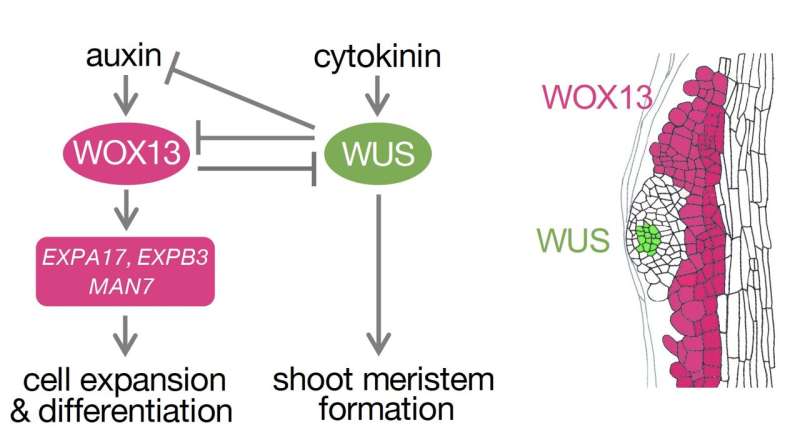
Plants have the unique ability to regenerate entirely from a somatic cell, i.e., an ordinary cell that does not typically participate in reproduction. This process involves the de novo (or new) formation of a shoot apical meristem (SAM) that gives rise to lateral organs, which are key for the plant's reconstruction.
At the cellular level, SAM formation is tightly regulated by either positive or negative regulators (genes/protein molecules) that may induce or restrict shoot regeneration, respectively. But which molecules are involved? Are there other regulatory layers that are yet to be uncovered?
To seek answers to the above questions, a research group led by Nara Institute of Science and Technology (NAIST), Japan studied the process in Arabidopsis, a plant commonly used in genetic research. Their research—which was published in Science Advances—identified and characterized a key negative regulator of shoot regeneration.
They demonstrated how the WUSCHEL-RELATED HOMEOBOX 13 (WOX13) gene and its protein can promote the non-meristematic (non-dividing) function of callus cells by acting as a transcriptional (RNA-level) repressor, thereby impacting regeneration efficiency.
"The search for strategies to enhance shoot regeneration efficiency in plants has been a long one. But progress has been hindered because the related regulatory mechanisms have been unclear. Our study fills this gap by defining a new cell-fate specification pathway," explains Momoko Ikeuchi, the principal investigator of this study.
Previous studies from the team had already established the role of WOX13 in tissue repair and organ adhesion after grafting. Hence, they first tested the potential role of this gene in the control of shoot regeneration in a wox13 Arabidopsis mutant (plant with dysfunctional WOX13) using a two-step tissue culture system.
Phenotypic and imaging analysis revealed that shoot regeneration was accelerated (three days faster) in plants lacking WOX13, and slower when WOX13 expression was induced. Moreover, in normal plants, WOX13 showed locally reduced expression levels in SAM. These findings suggest that WOX13 can negatively regulate shoot regeneration.
To validate their findings, the researchers compared the wox13 mutants and wild-type (normal) plants using RNA-sequencing at multiple time points. The absence of WOX13 did not considerably alter Arabidopsis gene expression under callus-inducing conditions. However, shoot-inducing conditions significantly enhanced the alterations induced by the wox13 mutation, leading to an upregulation of shoot meristem regulator genes.
Interestingly, these genes were suppressed within 24 hours of WOX13 overexpression in mutant plants. Overall, they found that WOX13 inhibits a subset of shoot meristem regulators while directly activating cell wall modifier genes involved in cell expansion and cellular differentiation. Subsequent Quartz-Seq2-based single cell RNA sequencing (scRNA-seq) confirmed the key role of WOX13 in specifying the fate of pluripotent callus cells.
This study highlights that unlike other known negative regulators of shoot regeneration, which only prevent the shift from callus toward SAM, WOX13 inhibits SAM specification by promoting the acquisition of alternative fates. It achieves this inhibition through a mutually repressive regulatory circuit with the regulator WUS, promoting the non-meristematic cell fate by transcriptionally inhibiting WUS and other SAM regulators and inducing cell wall modifiers.
In this way, WOX13 acts as a major regulator of regeneration efficiency. "Our findings show that knocking out WOX13 can promote the acquisition of shoot fate and enhance shoot regulation efficiency. This means that WOX13 knockout can serve as a tool in agriculture and horticulture and boost the tissue culture-mediated de novo shoot regeneration of crops," concludes Ikeuchi.
More information: Nao Ogura et al, WUSCHEL-RELATED HOMEOBOX 13 suppresses de novo shoot regeneration via cell fate control of pluripotent callus, Science Advances (2023). DOI: 10.1126/sciadv.adg6983. www.science.org/doi/10.1126/sciadv.adg6983
Journal information: Science Advances
Provided by Nara Institute of Science and Technology
















