This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The future of industrial chemicals: Engineers seek more efficient processes
A study by a team of University of Oklahoma researchers has been featured in Cell Reports Physical Science, an open-access journal highlighting cutting-edge research in the physical sciences.
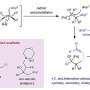
The study, "Cooperative roles of water and metal-support interfaces in the selective hydrogenation of cinnamaldehyde over cobalt boride catalysts," explores the role of water in the selective hydrogenation of carbonyl over alkene bonds. Utilizing cobalt and cobalt boride catalysts, OU researchers analyzed the hydrogenation of an organic compound called cinnamaldehyde. They discovered that the chemical element boron species plays a crucial role in enhancing catalytic activity and selectivity.
"We want to mimic nature's enzymes and learn more about what we can create synthetically. Our findings could have far-reaching implications in the production of industrial chemicals," said the project's co-investigator, Daniel Resasco, Ph.D., a professor in the School of Chemical, Biological and Materials Engineering, Gallogly College of Engineering.
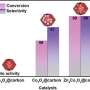
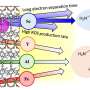
During thermal treatments, the boron species undergo a process called exsolution, where they separate from the bulk phase and accumulate on the catalyst's surface. This enrichment leads to the formation of acidic species, which boost the activity and selectivity of carbonyl bond hydrogenation by three and two times, respectively.
Resasco explains the significance of the team's findings. "The role of water in selective hydrogenation has long been a subject of interest. Our study provides new insights into the underlying mechanisms and uncovers a synergistic effect between boron species and water, ultimately leading to enhanced stability and selectivity of the catalysts," he said.
Associate professor Bin Wang, Ph.D., is a co-investigator on the project and says the study highlights the importance of finding the right balance between catalyst support and desired chemical outcomes. "This research opens new possibilities for developing more efficient and selective catalytic processes in the production of industrial chemicals," Wang said.
Resasco credits Li Gengnan, Ph.D., for the project's scientific thinking. Gengnan served as a post-doctorate fellow at OU before joining the Center for Functional Nanomaterials at Brookhaven National Laboratory, one of five Nanoscale Science Research Centers created by the Department of Energy.
"As the search for sustainable and efficient chemical processes continues, we hope to pave the way for transformative advancements in catalysis, driving us closer to a greener and more resourceful future," Resasco said.
More information: Gengnan Li et al, Cooperative roles of water and metal-support interfaces in the selective hydrogenation of cinnamaldehyde over cobalt boride catalysts, Cell Reports Physical Science (2023). DOI: 10.1016/j.xcrp.2023.101367
Journal information: Cell Reports Physical Science
Provided by University of Oklahoma