This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new bacterial blueprint to aid in the war on antibiotic resistance

A team of scientists from around the globe, including those from Trinity College Dublin, has gained high-res structural insights into a key bacterial enzyme that may help chemists design new drugs to inhibit it and thus suppress disease-causing bacteria. Their work is important as fears continue to grow around rising rates of antibiotic resistance.
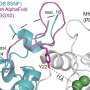
The scientists, led by Martin Caffrey, Fellow Emeritus in Trinity's School of Medicine and School of Biochemistry and Immunology, used next-gen X-ray crystallography and single particle cryo-electron microscopy techniques to "look under the bacterial bonnet" and produce a molecular blueprint of the full-length enzyme that may be used to design drugs that attack any structural weaknesses.
Because the enzyme Lnt is not found in humans—it only exists in bacteria and helps them build stable cell membranes through which things are transported in and out of cells—it is of huge potential significance as a therapeutic target as any bespoke drug designed to attack it should have fewer side-effects for patients. The research has just been published in the journal Science Advances.
Caffrey said, "A number of disease-causing bacteria have developed resistance to a plethora of first-choice drugs used to treat them and, with antimicrobial resistance on the rise in general, the World Health Organization has for some time now advised that a post-antibiotic era, in which minor injuries and common infections could prove fatal, is looming.
"New drugs are therefore badly needed and, while the journey can be a long one from providing a structural blueprint like this to developing a new drug, the precision to which we have resolved this potential target paints something of a 'bullseye' on that target."
More information: Luke Smithers et al, Structure snapshots reveal the mechanism of a bacterial membrane lipoprotein N-acyltransferase, Science Advances (2023). DOI: 10.1126/sciadv.adf5799. www.science.org/doi/10.1126/sciadv.adf5799
Journal information: Science Advances
Provided by Trinity College Dublin