This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
A simple model to evaluate skeletal muscle-macrophage interaction during skeletal muscle regeneration
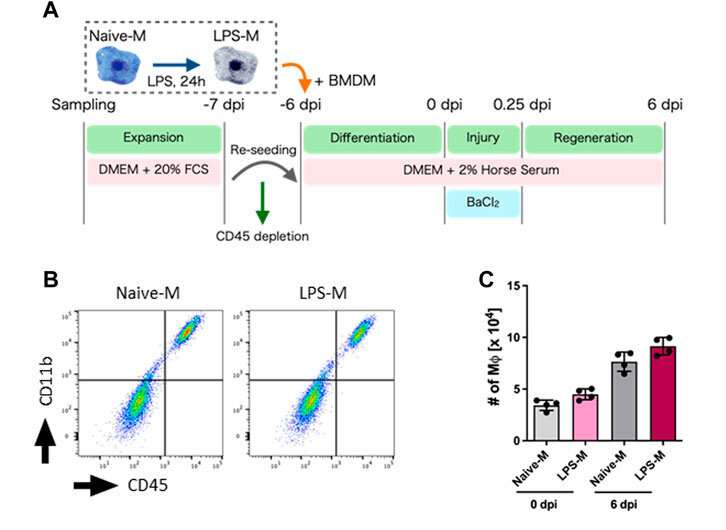
A research group led by Dr. Naoya Kase and Prof. Megumu Saito have successfully established a model to evaluate skeletal muscle regeneration efficiency and determine how macrophages contribute to the process by using a simple culture method that does not rely on special cell culture conditions. The results of this study were published online in Frontiers in Cell and Developmental Biology on May 18, 2023.
Muscle tissue has a high capacity to regenerate after injury. Macrophages, a type of immune cell, play a critical role in muscle regeneration, but the detailed mechanism by which they contribute to muscle regeneration is not fully understood.
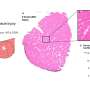
The research group created a simple model by culturing mouse primary muscle cells in a dish. The group found macrophages in the culture before and after muscle injury and that they contributed to muscle regeneration. Furthermore, the group observed macrophages pre-treated with lipopolysaccharide (LPS), a macrophage-activating molecule, had a lower capacity to help muscle regenerate due to increased extracellular matrix production.
Together, the researchers developed a new model to study the interactions between skeletal muscle cells and macrophages and assess how muscle regeneration may be affected. By identifying the mechanism by which macrophages contribute to muscle regeneration, it will be of tremendous interest to examine how this may change in various muscular pathologies. Moreover, these findings suggest macrophages could be a viable therapeutic target for muscle disorders and injuries.
More information: Naoya Kase et al, A concise in vitro model for evaluating interactions between macrophage and skeletal muscle cells during muscle regeneration, Frontiers in Cell and Developmental Biology (2023). DOI: 10.3389/fcell.2023.1022081
Provided by Kyoto University