This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists capture elusive chemical reaction using enhanced X-ray method

Researchers at SLAC National Accelerator Laboratory captured one of the fastest movements of a molecule called ferricyanide for the first time by combining two ultrafast X-ray spectroscopy techniques. They think their approach could help map more complex chemical reactions like oxygen transportation in blood cells or hydrogen production using artificial photosynthesis.
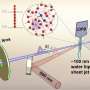
The research team from SLAC, Stanford and other institutions started with what is now a fairly standard technique: They zapped a mixture of ferricyanide and water with an ultraviolet laser and bright X-rays generated by the Linac Coherent Light Source (LCLS) X-ray free-electron laser. The ultraviolet light kicked the molecule into an excited state while the X-rays probed the sample's atoms, revealing features of ferricyanide's atomic and electronic structure and motion.
What was different this time is how the researchers extracted information from the X-ray data. Instead of studying only one spectroscopic region, known as the Kβ main emission line, the team captured and analyzed a second emission region, called valence-to-core, which has been significantly more challenging to measure on ultrafast timescales. Combining information from both regions enabled the team to obtain a detailed picture of the ferricyanide molecule as it evolved into a key transitional state.
The team showed that ferricyanide enters an intermediate, excited state for about 0.3 picoseconds—or less than a trillionth of a second—after being hit with a UV laser. The valence-to-core readings then revealed that following this short-lived, excited period, ferricyanide loses one of its molecular cyanide "arms," called a ligand. Ferricyanide then either fills this missing joint with the same carbon-based ligand or, less likely, a water molecule.
"This ligand exchange is a basic chemical reaction that was thought to occur in ferricyanide, but there was no direct experimental evidence of the individual steps in this process," SLAC scientist and first author Marco Reinhard said. "With only a Kβ main emission line analysis approach, we wouldn't really be able to see what the molecule looks like when it is changing from one state to the next; we'd only obtain a clear picture of the beginning of the process."
"You want to be able to replicate what nature does to improve technology and increase our foundational scientific knowledge," SLAC senior scientist Dimosthenis Sokaras said. "And in order to better replicate natural processes, you have to know all of the steps, from the most obvious to those that happen in the dark, so to speak."
In the future, the research team wants to study more complex molecules, such as hemeproteins, which transport and store oxygen in red blood cells—but which can be tricky to study because scientists do not understand all the intermediate steps of their reactions, Sokaras said.
The research team refined their X-ray spectroscopy technique at SLAC's Stanford Synchrotron Radiation Lightsource (SSRL) and LCLS over many years, and then combined all this expertise at LCLS's X-ray Correlation Spectroscopy (XCS) instrument to capture the molecular structural changes of ferricyanide. The team published their results today in Nature Communications.
"We leveraged both SSRL and LCLS to complete the experiment. We couldn't have finished developing our method without access to both facilities and our longstanding collaboration together," said Roberto Alonso-Mori, SLAC lead scientist. "For years, we have been developing these methods at these two X-ray sources, and now we plan to use them to uncover previously inaccessible secrets of chemical reactions."
More information: Marco Reinhard et al, Ferricyanide photo-aquation pathway revealed by combined femtosecond Kβ main line and valence-to-core x-ray emission spectroscopy, Nature Communications (2023). DOI: 10.1038/s41467-023-37922-x
Journal information: Nature Communications
Provided by SLAC National Accelerator Laboratory