This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
After spinal cord injury, kinesthetic sense helps restore movement, model suggests

For nearly 50 years, a jawless fish called the lamprey has interested scientists because of its remarkable ability to recover from spinal cord injuries. A new study reveals a possible technique lampreys may use to swim again, despite sparse neural regeneration.
Christina Hamlet of Bucknell University and collaborators, including Jennifer R. Morgan of the Marine Biological Laboratory (MBL), used a mathematical model to demonstrate how lampreys may use body-sensing feedback to regain swimming abilities after spinal injury. The study could inspire new therapeutic approaches in humans or algorithms for locomotion in soft robots. The paper is published in Proceedings of the National Academy of Sciences.
"The punchline of the paper is that even in the absence of descending command across that [spinal] lesion, you can boost the sensory feedback and restore locomotion," said Morgan, MBL Senior Scientist and Director of the MBL's Eugene Bell Center for Regenerative Biology and Tissue Engineering.
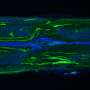
Unlike humans and other mammals, lampreys recover quickly and almost completely even after severe lesions high up in the spinal cord. Morgan previously discovered that although neural regeneration does aid recovery in lampreys, it doesn't tell the full story. Only a small percentage of the neurons and neuronal connections are restored across a spinal injury, so they must use another mechanism.
"I had all of these questions about how that could possibly work. How could you get a functioning nervous system with a few small sparse connections?" Morgan asked.
Scientists had hypothesized that lampreys might use body-sensing feedback (called proprioception or kinesthesia) to guide their movements in addition to descending neural connections in the spinal cord. Morgan had reached out to discuss this with an old friend of hers from MBL, Eric Tytell, Associate Professor of Biology at Tufts University and former MBL Whitman Center Investigator. Eric was already collaborating with Lisa Fauci, a Professor of Mathematics at Tulane University, and Christina Hamlet, who was a co-mentored postdoc at Tulane.
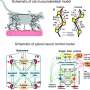
Tytell, Fauci, and Hamlet were using mathematical models to mimic movement in lampreys. They teamed up to "see if we could model some of the effects of sensory feedback on swimming behavior in lampreys," said Hamlet, who is currently an Assistant Professor of Mathematics at Bucknell University.
The team began playing around with different scenarios of spinal injured lampreys—including both biologically plausible and implausible ones—all of which assumed no neural regeneration across the spinal cord lesion. This is the utility of modeling, said Hamlet, "We can break things that you can't break in biology." The model took into account the curves and stretch created in the body above the lesion and sent that information to the rest of the body through the muscles, not the spinal cord.
Even with a moderate amount of sensory feedback, the models showed a surprising recovery of swimming patterns in the biologically plausible models. Stronger sensory feedback led to even greater improvement.
Because lampreys do grow back some of their neurons after a lesion and therefore have descending command from the brain to drive movement, they may need even less sensory feedback than the model. The team hopes to add neuronal regeneration into the model and test how that affects movement and interacts with sensory feedback.
"If you have a good computational model, you can go through so many more scenarios of manipulations than is practical with experimentation," said Morgan.
The team hopes that this study and future research will contribute to therapies for humans with spinal injuries and diseases that affect movement. Brain machine interfaces and stimulator devices are beginning to incorporate body sensing feedback to create smoother movements after injury, and this research could inform the amount and type of feedback that humans need.
"Whether you're an animal like a lamprey that [recovers] spontaneously or a human that needs to be given a drug or an electrical stimulator device, getting to the point where you have a few things in the right place and then reuse what's already there should be more achievable than trying to recapitulate the identical original pattern of synaptic connections and growth," said Morgan.
More information: Christina Hamlet et al, Proprioceptive feedback amplification restores effective locomotion in a neuromechanical model of lampreys with spinal injuries, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2213302120
Journal information: Proceedings of the National Academy of Sciences
Provided by Marine Biological Laboratory