This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists discover a way Earth's atmosphere cleans itself

Human activities emit many kinds of pollutants into the air, and without hydroxyl radicals (OH), many of these pollutants would keep aggregating in the atmosphere.
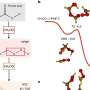
How OH itself forms in the atmosphere was viewed as a complete story, but in new research published in Proceedings of the National Academy of Sciences, a research team that includes Sergey Nizkorodov, a University of California, Irvine professor of chemistry, report that a strong electric field that exists at the surface between airborne water droplets and the surrounding air can create OH by a previously unknown mechanism.
It's a finding that stands to reshape how scientists understand how the air clears itself of things like human-emitted pollutants and greenhouse gases, which OH can react with and eliminate. "You need OH to oxidize hydrocarbons, otherwise they would build up in the atmosphere indefinitely," said Nizkorodov.
"OH is a key player in the story of atmospheric chemistry. It initiates the reactions that break down airborne pollutants and helps to remove noxious chemicals such as sulfur dioxide and nitric oxide, which are poisonous gases, from the atmosphere," said Christian George, an atmospheric chemist at the University of Lyon in France and lead author of the new study. "Thus, having a full understanding of its sources and sinks is key to understanding and mitigating air pollution."
Before, researchers assumed that sunlight was the chief driver of OH formation.
"The conventional wisdom is that you have to make OH by photochemistry or redox chemistry. You have to have sunlight or metals acting as catalysts," Nizkorodov said. "What this paper says in essence is you don't need any of this. In the pure water itself, OH can be created spontaneously by the special conditions on the surface of the droplets."
The team built on research from Stanford University scientists led by Richard Zare that reported spontaneous formation of hydrogen peroxide on the surfaces of water droplets. The new findings help interpret the unexpected results from the Zare group.
The team measured OH concentrations in different vials—some containing an air-water surface and others containing only water without any air—and tracked OH production in darkness by including a "probe" molecule in the vials that fluoresces when it reacts with OH.
What they saw is that OH production rates in darkness mirror those and even exceed rates from drivers like sunlight exposure. "Enough of OH will be created to compete with other known OH sources," said Nizkorodov. "At night, when there is no photochemistry, OH is still produced and it is produced at a higher rate than would otherwise happen."
The findings, Nizkorodov reported, alter understanding of the sources of OH, something that will change how other researchers build computer models that attempt to forecast how air pollution happens.
"It could change air pollution models quite significantly," Nizkorodov said. "OH is an important oxidant inside water droplets and the main assumption in the models is that OH comes from the air, it's not produced in the droplet directly."
To determine whether this new OH production mechanism plays a role, Nizkorodov thinks the next step is to perform carefully designed experiments in the real atmosphere in different parts of the world.
But first, the team expects the results to make a splash in the atmospheric research community.
"A lot of people will read this but will not initially believe it and will either try to reproduce it or try to do experiments to prove it wrong," said Nizkorodov. "There will be many lab experiments following up on this for sure."
He added that UCI is a prime place for such science to continue happening, because other labs at UCI, like that of Ann Marie Carlton, professor of chemistry, focus their efforts on the role water droplets play in the atmosphere.
More information: Kangwei Li et al, Spontaneous dark formation of OH radicals at the interface of aqueous atmospheric droplets, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2220228120
Journal information: Proceedings of the National Academy of Sciences
Provided by University of California, Irvine