This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Towards more efficient and eco-friendly thermoelectric oxides with hydrogen substitution
Today, over half of the total energy produced from fossil fuels is discarded as waste heat, which accelerates global warming. If we could convert the waste heat into a more useful form of energy like electricity, we could minimize fuel consumption and reduce our carbon footprint. In this regard, thermoelectric energy conversion has gained momentum as a technology for generating electricity from waste heat.
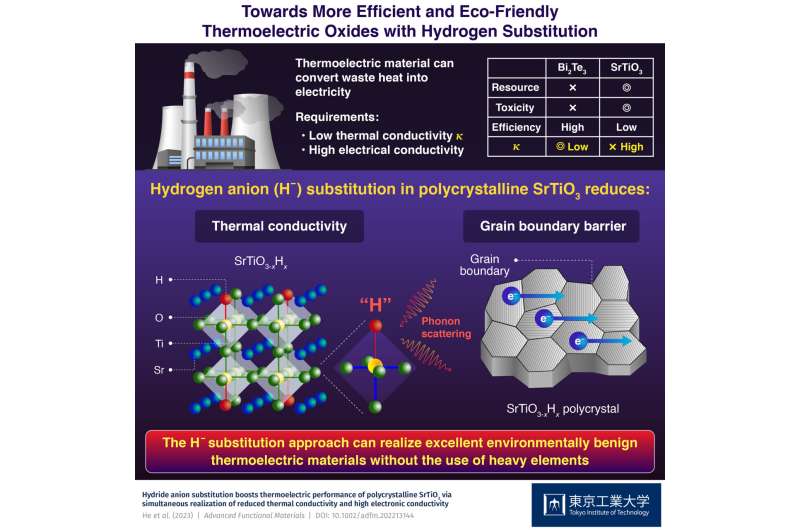
For efficient conversion, a thermoelectric material must have a high conversion efficiency (ZT). So far, realizing a high ZT has been possible only with the use of heavy elements like lead, bismuth, and tellurium. However, the use of rare, expensive, and environmentally toxic elements such as these has limited the large-scale application of thermoelectric energy conversion.
To tackle these issues, transition metal oxides based on platforms such as SrTiO3 have emerged as a more inexpensive and benign alternative. However, their ZT is typically limited by a high thermal conductivity (κ), since for a high κ, the temperature across the material becomes uniform more quickly, and the lowered temperature difference—the driving force behind the thermoelectric conversion—causes electric power generation to decrease as well.
Against this backdrop, a research team including Associate Professor Takayoshi Katase from Tokyo Institute of Technology (Tokyo Tech), Japan recently discovered a new approach to reducing κ and boosting the performance of SrTiO3 by hydrogen substitution.
Conventionally, the use of light elements is expected to increase the κ originating from lattice vibration (κlat), leading to the adoption of heavy elements to reduce the κlat. In contrast, in their study published in Advanced Functional Materials, the team discovered that the κlat of SrTiO3 could be reduced to less than half its original value by substituting a light element, namely hydrogen.
They clarified the mechanism underlying their observation using first-principles calculations, which showed that substituting a portion of the oxygen anions (O−) with hydrogen anions (H−), yielding compounds of the form SrTiO3−xHx, results in a mixture comprising a strong Ti-O bond and a weak Ti-H bond. These randomly distributed Ti-(O,H) bonds, in turn, largely decrease κlat.
The team also found that SrTiO3−xHx polycrystals exhibit high electron mobility comparable to that of single-crystal materials without any deterioration in electron conduction across grain boundaries. Based on these two effects, a low thermal conductivity along with a high electrical output power are realized at the same time, resulting in an improved thermoelectric conversion efficiency in the SrTiO3−xHx polycrystal.
Overall, these findings can open doors to innovative strategies for developing next-generation thermoelectric materials. "In future, the hydrogen substitution approach would realize excellent environmentally benign thermoelectric materials that do not require the use of heavy elements," concludes Dr. Katase.
More information: Xinyi He et al, Hydride Anion Substitution Boosts Thermoelectric Performance of Polycrystalline SrTiO 3 via Simultaneous Realization of Reduced Thermal Conductivity and High Electronic Conductivity, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202213144
Journal information: Advanced Functional Materials
Provided by Tokyo Institute of Technology