This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Easy and quick binding of targeting molecule and radiotracer to drug nanocarrier for cancer therapy
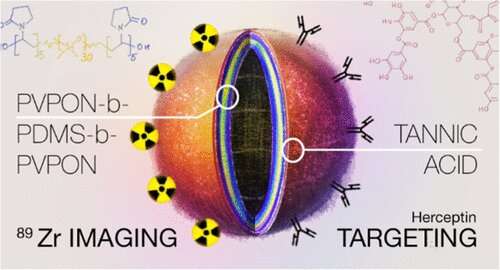
An ideal nanovesicle to fight cancer would have three functionalities: 1) a precision-targeting molecule to preferentially bind it to surface markers on cancer cells, 2) a strongly bound radionuclide signal that would allow a PET scan to locate the vesicles in the body, and 3) the ability to carry and release a drug treatment, such as a chemotherapy, at the cancer tumor.
It would also meet two other requirements—having a simple and facile method of manufacture, and being biocompatible and biodegradable in the body.
A University of Alabama at Birmingham team has now described a tiny polymersome that—in initial preclinical experiments—appears to meet these hurdles. Each polymersome is a hollow sphere with a thin wall, but it is the coating on the polymersome that marks a step forward.
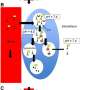
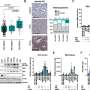
The 60-nanometer triblock copolymer polymersomes have degradable tannic acid, or TA, adsorbed onto the surface through hydrogen bonding. That TA, in turn, is able to quickly and stably bind a monoclonal antibody-targeting molecule and zirconium radionuclide, or Zr, without the need to build specific linkers, such as chelators, say Eugenia Kharlampieva, Ph.D., and Suzanne E. Lapi, Ph.D., leaders of the UAB team. Their study is published in the journal Biomacromolecules.
"In this study, we have developed a simple approach for a chelator-free modification of the PVPON5-PDMS30-PVPON5 triblock copolymer nanovesicles, about 60 to 80 nanometers in diameter, with a layer of polyphenol that can be simultaneously used to anchor 89Zr radiotracer or other active metal ions for molecular imaging, and a HER2-targeting ligand, trastuzumab monoclonal antibody, for nanovesicle targeting to HER2-positive breast cancer cells," said Kharlampieva, a distinguished professor in the UAB College of Arts and Sciences' Department of Chemistry. PVPON5 is a short, five-monomer hydrophilic polymer block, and PDMS30 is a longer, 30-monomer, hydrophobic polymer block within the triblock copolymer.
Breast cancer is one of the most common cancer diseases, and global rates of death are still high. Systemic drugs or therapeutic antibodies are current therapies, but they are often associated with heart damage and dysfunction. Image-guided drug delivery to a solid tumor could allow effective drug activity with reduced drug toxicity.
"To the best of our knowledge, our work represents the first example of a chelator-free-radiolabeled polymersome capable of a long-term multiday PET imaging study in vivo," said Lapi, director of the UAB Cyclotron Facility and a professor in the UAB Department of Radiology. "The radiolabeling approach developed herein can potentially provide stable binding of a wide spectrum of isotopes without radiometal leaching from the vesicle membrane in vivo. Notably, this approach integrates the inherent advantages of a polyphenolic polymersome membrane with the benefit of quickly anchoring breast cancer cell-targeting ligands."
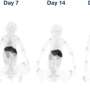
In the study, TA on the polymersome bound 89Zr4+ radionuclide through nonspecific ionic pairing, and the TA also bound the trastuzumab monoclonal antibody, or Tmab, through hydrogen bonding and ionic pairing. There was excellent retention of the 89Zr for up to seven days, as confirmed by PET scans in healthy mice.
"The noncovalent Tmab anchoring to the polymersome membrane can be highly advantageous for nanoparticle modification compared to currently developed covalent methods, as it allows easy and quick integration of a broad range of targeting proteins," Kharlampieva said. "Given the ability of these polymersomes to encapsulate and release anticancer therapeutics, they can be further expanded as precision-targeted therapeutic carriers for advancing human health through highly effective drug-delivery strategies."
One hour of incubating the TA-polymersomes in a solution of 89Zr-oxalate led to radiolabeling yields of 97 percent, and those yields remained consistent over one, three and seven days. The labeled polymersomes were not cytotoxic when incubated in vitro with two lines of cancer cells up to four days. Furthermore, binding of 89Zr to polymersomes with Tmab already attached also had high yields of 97 percent and stability through seven days. These binding yields are sufficiently high for clinical use, the UAB researchers say.
Next, the stable retention of the 89Zr on the TA-polymersomes was demonstrated indirectly in mice.
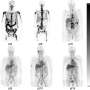
The biodistribution of free 89Zr radiotracer has been previously reported to localize largely in the spine and femurs of animals due to chelation of the zirconium with phosphate moieties in the bone. The UAB researchers found that, when free 89Zr was injected into mice, nearly all of it was located in the femur bones after 24 hours, as measured by a PET scan. A greatly different biodistribution was seen when 89Zr-TA-polymersomes were injected into mice. Negligible radioactivity was found in bones; instead, nearly all the radioactivity was in the spleen and liver. That location represents the known expected clearance of nanovesicles through the mononuclear phagocyte system for nanomaterials larger than 6 to 8 nanometers.
"The observed drastic difference between the biodistribution of the free 89Zr and the metal radiotracer-labeled vesicle is important, as it demonstrates an unimpeded capability of the polymeric nanocarrier to be tracked in vivo," Lapi said.
The strong imaging contrast in the mice was retained through seven days, further evidence of tight retention of the 89Zr on the TA-polymersomes.
The ability of the 89Zr-Tmab-TA-polymersomes to target HER2-positive cancer cells was shown in vitro by differential binding of the nanovesicles to HER2-positive breast cancer cells versus HER2-negative cells. The researchers say further testing to target breast cancer tumors in animals is warranted.
Co-first authors of the study, "Direct radiolabeling of trastuzumab-targeting triblock copolymer vesicles with 89Zr for positron emission tomography imaging," are Veronika Kozlovskaya, UAB Department of Chemistry, and Maxwell Ducharme, UAB Department of Radiology.
Other co-authors with Kharlampieva, Lapi, Kozlovskaya and Durcharme are Maksim Dolmat and James M. Omweri, UAB Department of Chemistry; and Volkan Tekin, UAB Department of Radiology.
More information: Veronika Kozlovskaya et al, Direct Radiolabeling of Trastuzumab-Targeting Triblock Copolymer Vesicles with 89Zr for Positron Emission Tomography Imaging, Biomacromolecules (2023). DOI: 10.1021/acs.biomac.2c01539
Journal information: Biomacromolecules
Provided by University of Alabama at Birmingham