This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists reveal protein synthesis mechanism in Giardia
Scientists from Uppsala University have used cryo-electron microscopy to reveal details of the protein synthesis mechanism in the parasite Giardia intestinalis, which causes diarrheic disease. The new insights could be valuable for screening specific drugs against Giardia and other protozoan parasites.
Cryo-electron microscopy (cryo-EM) is a very powerful tool that makes it possible to visualize cellular machineries with great magnification. The cryogenic set-up prevents damage and distortion of the biological samples and as a result the cellular processes can be viewed as a series of snapshots, but with molecular details.
In recent years cryo-EM has been instrumental in unraveling the steps of protein synthesis in various species, from bacteria to humans. Yet, there was no knowledge of the protein synthesis process in protozoan organisms, a group of single-celled organisms that are often parasitic to humans and other mammals.
Uppsala scientist Professor Suparna Sanyal and her team members, particularly Dr. Soneya Majumdar and Ph.D. student Andrew Emmerich, took up the challenging project of unraveling the details of protein synthesis of Giardia intestinalis in 2020.
Giardia is an important research topic, not just because it causes nasty diarrhea in mammals, but also because it serves as a model system for much more hazardous protozoan parasites. In collaboration with Giardia expert Professor Staffan Svärd at Uppsala University, the research group cultured Giardia and purified native ribosomes—which are the protein factory of the cell. They set up cryo-EM grids in the facility at Uppsala University and collected cryo-EM data in the Umeå node of the SciLifeLab facility.
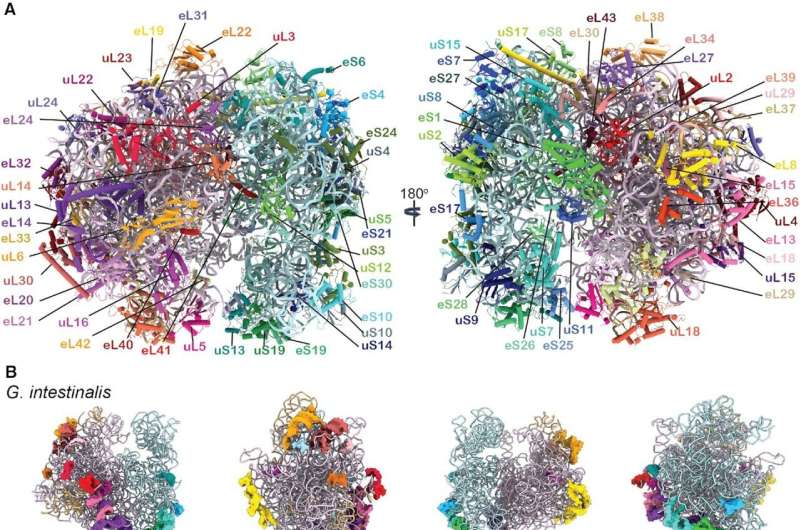
After two years of hard work, they were able to resolve high-resolution structures of six different states of elongation of Giardia protein synthesis. These novel structures could be assembled in a video showing sequential changes in the ribosome structure, movement of the tRNAs, and the binding and release of translation elongation factor eEF2—all essential for protein synthesis in Giardia and other protozoa.
Moreover, Sanyal and colleagues could decipher important differences of the Giardia ribosome from bacterial and human ribosomes, which could be valuable for screening specific drugs against Giardia and other protozoan parasites.
The findings are published in the journal Nucleic Acids Research.
More information: Soneya Majumdar et al, Insights into translocation mechanism and ribosome evolution from cryo-EM structures of translocation intermediates of Giardia intestinalis, Nucleic Acids Research (2023). DOI: 10.1093/nar/gkad176
Journal information: Nucleic Acids Research
Provided by Swedish Research Council