This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Investigators identify new pattern recognition system that monitors disease-causing bacteria in C. elegans
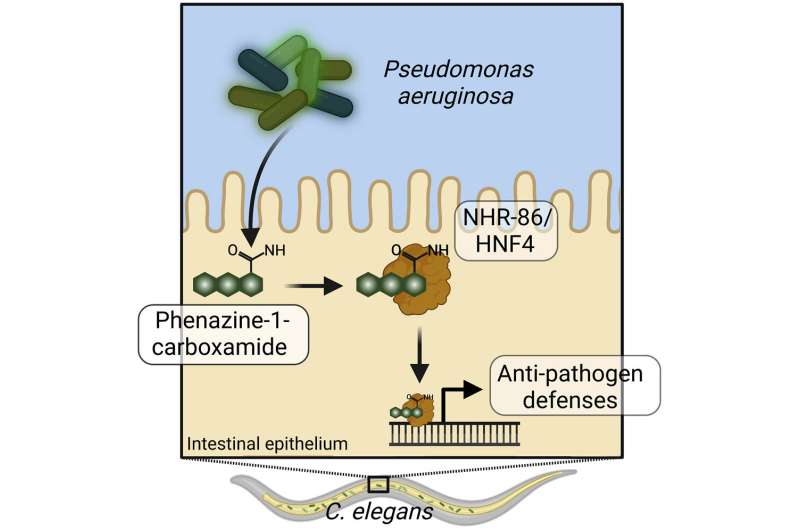
A study published in Immunity by physician-scientist Read Pukkila-Worley, MD, and MD/Ph.D. students Nicholas D. Peterson and Samantha Y. Tse describes a new manner of detecting microbial infection that intercepts pathogen-derived signals of growth to assess the relative threat of virulent bacteria.
A nuclear hormone receptor in the nematode C. elegans senses a toxic metabolite produced by the bacterial pathogen Pseudomonas aeruginosa to activate innate immunity. These data reveal an ancient strategy that informs the origins of pathogen detection and may be among the most primordial forms of immune sensing in animals.
"Our research adds to our understanding of how hosts differentiate between beneficial and harmful bacteria, which teaches us something important about how our immune systems evolved," said Dr. Pukkila-Worley, associate professor of medicine.
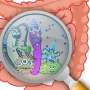
Distinguishing potentially harmful pathogens from benign microorganisms is one of the primary functions of the innate immune system in all animals. This is particularly important for nematodes, such as C. elegans—the transparent microscopic worm often used as a model organism to study genetics and gene function—that consume bacteria as their food source.
Working with Pseudomonas aeruginosa, a bacteria that commonly infects immune-compromised patients in the hospital and is increasingly resistant to standard antibiotic treatments, Pukkila-Worley and colleagues performed a series of genetic screens with mutant bacteria, one-by-one, to see if any impacted the innate immune system response in C. elegans.
They found that bacteria that cannot produce a specific phenazine metabolite were able to avoid detection by the innate immune system, suggesting that the bacterial phenazine metabolite was sensed to activate innate immunity.
"This result was intriguing because P. aeruginosa use phenazines for growth and virulence. Thus, the innate immune system can intercept signals produced by bacteria in order to identify bacteria that have grown to dangerous levels and are poised to cause disease," said Pukkila-Worley.
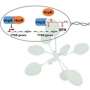
Researchers in the Pukkila-Worley lab designed a second experiment to identify the sensor in the host that detects these phenazine metabolites. They discovered that a specialized type of transcription factor, a nuclear hormone receptor, binds the phenazine metabolite and directly activates anti-pathogen defenses.
"One of the striking things about our results is that C. elegans senses this bacterial metabolite to detect an individual bacterial pathogen in a remarkably specific manner from among its bacterial food," said Peterson, an MD/Ph.D. student in the Pukkila-Worley lab.
In humans, pattern-recognition systems in the intestine involving Toll-like receptors scan the physical structure of different bacteria to sense the presence of infectious microorganisms. Nematodes lost pattern-recognition receptors in evolution. Pukkila-Worley and colleagues show that nematodes use nuclear hormone receptors to detect specific pathogen-derived metabolites to activate innate immunity, which represents a new type of pattern-recognition.
Since C. elegans have 274 nuclear hormone receptors, it's possible that the nematode genome contains dozens of these metabolite recognition systems. Nuclear hormone receptors are also found in most animals, including humans, suggesting that similar metabolite detection systems might exist in other organisms.
"It's remarkable that C. elegans evolved mechanisms to differentiate good and bad bacteria even without canonical receptors for pathogen detection. This further supports the importance of understanding how our immune system evolved over time to deepen our understanding of host-microbiome interactions," said Tse, an MD/Ph.D. student in the Pukkila-Worley lab.
More information: Nicholas D. Peterson et al, Non-canonical pattern recognition of a pathogen-derived metabolite by a nuclear hormone receptor identifies virulent bacteria in C. elegans, Immunity (2023). DOI: 10.1016/j.immuni.2023.01.027
Journal information: Immunity
Provided by UMass Chan Medical School