This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New targeting opportunities discovered against canine distemper virus
The highly contagious canine distemper virus is dangerous to dogs and wild life animals. It is also closely related to the equally highly contagious measles virus. Researchers at the University of Bern and the Zurich University of Applied Sciences have now for the first time determined the structure of the canine distemper virus "docking protein" and depicted it at molecular level. This lays the ground to develop novel therapies for a better management of the diseases induced by CDV and related viruses, such as the measles virus.
Measles virus and canine distemper virus (CDV) belong to the genus Morbillivirus. Both infectious pathogens are highly contagious RNA viruses surrounded by an envelope on which their "docking proteins" protrude—similar to the spike protein in coronavirus. Both viruses induce respiratory infections as well as deadly encephalitis, although high incidence of brain infections is unique to CDV.
Despite the availability of an efficient vaccine, measles still kills more than 100,000 people each year. Canine distemper virus, for its part, causes large epidemics, especially in wild life animals, including endangered species such as certain tiger species. There is also a high risk of spillover to other animal species—in countries associated with suboptimal vaccine coverage, the virus can severely affect dogs.
No antiviral drug currently approved
For measles, antivirals would act as an attractive complement to vaccination campaigns. And for CDV, antiviral drugs may support disease management in susceptible endangered species that are in captivity, e.g., Pandas. However, no antiviral morbillivirus drug is currently approved.
To develop effective antiviral drugs, a better understanding of the structure of the measles and canine distemper viruses and the mechanisms that allow them to enter human and animal cells is needed.
Researchers led by Dimitrios Fotiadis of the Institute of Biochemistry and Molecular Medicine (IBMM) of the Medical Faculty of the University of Bern and Philippe Plattet of the Division of Neurological Sciences of the Vetsuisse Faculty of the University of Bern have now succeeded for the first time in determining the structure of the "docking protein" of the canine distemper virus and depicting it at molecular level.
These findings make it possible to develop "tailor-made" active substances against the "docking protein" that prevent the virus from entering host cells. The study was published in the journal Proceedings of the National Academy of Sciences.
Targeted blocking of the 'docking protein'
The mechanism by which measles and canine distemper viruses enter cells relies on two proteins on the viral envelope: a "docking protein" (also called H-protein) and a "fusion protein" (F-protein). Based on previous research, it is assumed that upon interaction with a host cell receptor, the H-protein transmits a signal that will activate the F-protein.
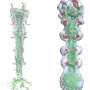
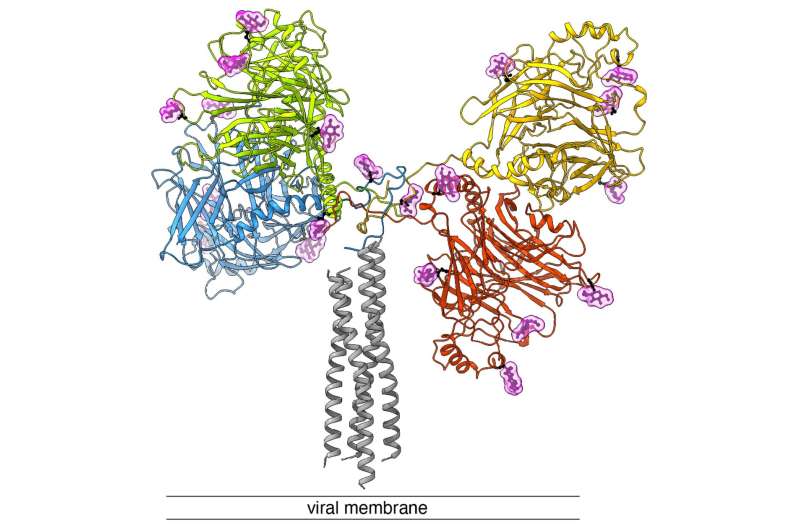
This results in a fusion of the viral envelope with the host cell membrane. In this process, a so-called fusion pore is formed, which enables the injection of the viral genome into the host cell. Now, the team led by Dimitrios Fotiadis and Philippe Plattet, together with researchers from the Zurich University of Applied Sciences (ZHAW), have been able to determine the structure of this H-protein for the first time using cryo-electron microscopy (cryo-EM) and map it at molecular level.
In cryo-electron microscopy, biological samples are imaged at cryogenic temperatures (around -180 °C) and magnified by 100,000 times. This revealed that the protein is characterized by three main domains (heads, neck and stalk) that form a "Y." "The fact that we were able to determine the structure represents a big leap forward.
This now allows us to understand how the different subdomains spatially organize with each other—and provides us with a valuable blueprint to develop next-generation antiviral drugs that block the 'docking protein'," says Dimitrios Fotiadis.
Novel therapeutic approaches
"Simultaneously blocking the cell entry process in distemper and measles virus with several different neutralizing molecules is a promising antiviral strategy," explains Philippe Plattet. Currently, researchers from the Plattet and Fotiadis consortium and the University of Marseille have successfully identified antibodies that neutralize CDV in a highly effective manner.
In further research, the recently established cryo-EM platform at the University of Bern will provide useful services: structural studies for CDV and related viruses can now be extended and accelerated, for example to determine the structures of the H-proteins of measles and distemper viruses when bound to neutralizing antibodies. "Thanks to the viral structures determined by cryo-EM, we can develop and improve antiviral drugs using so-called structure-based drug design," says Fotiadis.
More information: David Kalbermatter et al, Structure and supramolecular organization of the canine distemper virus attachment glycoprotein, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2208866120
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Bern