This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers identify gene mutation capable of regulating pain
Pain afflicts at least 1.5 billion people worldwide, and despite the availability of various painkilling drugs, not all forms of pain are treatable. Moreover, pain medications can have side-effects such as dependence and tolerance, especially in the case of morphine and other opioids.
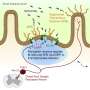
In search of novel painkillers, researchers at Butantan Institute's Special Pain and Signaling Laboratory (LEDS) in São Paulo, Brazil, studied TRPV1, a sensory neuron receptor that captures noxious stimuli, including heat and the burning sensation conveyed by chili peppers, and discovered a potential pain insensitivity mutation in the gene that encodes this protein. They report their findings in an article published in the Journal of Clinical Investigation.
The study was conducted in partnership with Stanford University and Emory University in the United States, and Münster University Hospital in Germany. The researchers analyzed a number of mutations in humans and also benefited from existing knowledge of birds, which unlike mammals have a TRPV1 receptor that is naturally resistant to noxious insults and even peppery food, yet can still perceive pain.
"There are more than 1,000 TRPV1 mutations in humans, and there's nothing novel about trying to switch the receptor off in order to relieve pain, but these attempts haven't been successful until now," said Vanessa Olzon Zambelli, a researcher at LEDS and co-first author of the article.
"First, many drugs resulting from this process interfere with body temperature regulation. Second, TRPV1 is an important channel for signaling heat, and completely altering its activity cancels out physiological pain, interfering with the sensation of burning heat, which has a protective function."
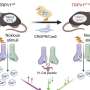
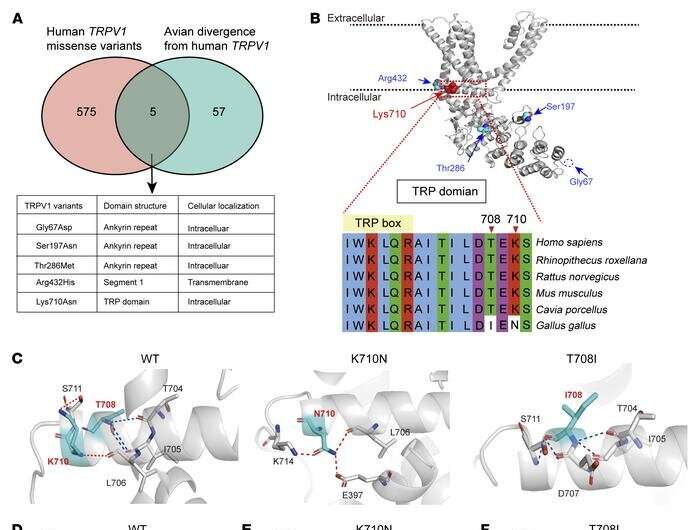
The researchers began by exploring a genome database to compare the genetic sequences of avian and human TRPV1. Using a computational approach, they identified five avian mutations they believed to be linked to resistance to pain.
Cryogenic electron microscopy (which does not require large sample sizes or crystallization and is therefore suited to the visualization of structures at near-atomic resolution) showed that the five avian mutations were located in K710, an amino acid residue believed to control gating (opening and closing) of the TRPV1 channel.
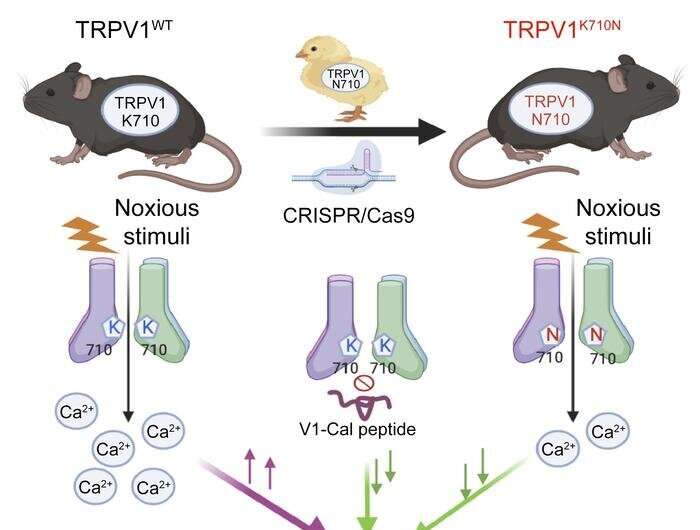
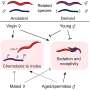
The mutations can also be present in humans, but they are very rare, so the researchers decided to find out what would happen if they were "transplanted" into mammals. When they tested these variants in genetically modified cells, they found that the function of the channel was indeed altered. Next, they used the CRISPR/Cas9 gene editing technique to create mice with the mutation K710N, which they had previously found to reduce the receptor's reaction to capsaicin in cells. Capsaicin is the active principle in pepper.
The researchers did not observe nociceptive behavior (suggesting avoidance of pain) in mice with the K710N mutation injected with capsaicin and given peppery chicken feed, in contrast with the behavior of normal mice, which lifted their paws to avoid touching the capsaicin, presumably because even skin contact caused pain.
The mice with the K710N mutation also showed less hypersensitivity to nerve injury, while their response to noxious heat remained intact. Furthermore, blocking the K710 region in normal mice limited acute behavioral responses to noxious stimuli and returned pain hypersensitivity induced by nerve injury to baseline levels.
In addition to modulating pain, TRPV1 also plays an important role in protection against other stimuli. For example, recent evidence suggests that it serves in non-neuronal cells as an intracellular molecular sensor that protects against glucose-induced cellular stress or tissue ischemia. Additional tests performed as part of this study involving cardiomyocytes (heart muscle cells) insulted with hydrogen peroxide, high levels of glucose and a cerebral ischemia model confirmed the protective effect even with the mutation.
Translational analysis
The second part of the study consisted of an attempt to reduce the receptor's function pharmacologically. To this end, the researchers developed a peptide, V1-cal, which acted selectively on the K710 region. Mice treated with V1-cal and given capsaicin displayed less nociceptive behavior and diminished release of neuropeptides leading to neurogenic inflammation and edema without altering temperature. Lastly, chronic pain also improved considerably.
"We now want to add value to this study by validating the results under best-practice laboratory conditions [required by regulatory agencies], identify other small molecules besides the peptide that can more easily be synthesized, conduct preclinical trials and, if these are successful, begin a clinical trial," Zambelli said.
More information: Shufang He et al, A human TRPV1 genetic variant within the channel gating domain regulates pain sensitivity in rodents, Journal of Clinical Investigation (2022). DOI: 10.1172/JCI163735
Journal information: Journal of Clinical Investigation
Provided by FAPESP