This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers uncover physics involved in a key process in Huntington's disease
Researchers from Princeton University have uncovered the physics of a cellular process linked to aggregation diseases, including Huntington's disease, paving the way to a deeper understanding of neurodegenerative disorders at the molecular level.
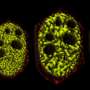
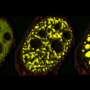
Many critical systems within a cell function inside liquid droplets that are separated from their surrounding fluids the way oil gathers in water. Scientists have only recently realized the importance of these droplets, especially in the kinds of processes that lead to cell breakdown and death.
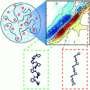
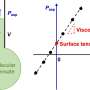
The Princeton-led team found that as such droplets drift in their surrounding liquid, they collide and gather into larger droplets in mathematically predictable ways. The math that describes this aggregation is similar to how internet memes go viral or scientific papers accumulate large numbers of citations. Abnormally large droplets in brain cells are linked closely to ALS, Alzheimer's and a range of other dysfunctions.
The work was led by Princeton faculty members Ned Wingreen, the Howard A. Prior Professor in the Life Sciences, and Cliff Brangwynne, the June K. Wu '92 Professor in Engineering. Brangwynne described the work as "important for establishing a quantitative framework for understanding the fundamental physical mechanism behind protein aggregation, a necessary step for developing treatments for these devastating diseases."
The paper is published in the journal Nature Physics.
More information: Daniel S. W. Lee et al, Size distributions of intracellular condensates reflect competition between coalescence and nucleation, Nature Physics (2023). DOI: 10.1038/s41567-022-01917-0
Journal information: Nature Physics
Provided by Princeton University