This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Scientists reveal flaws in tuberculosis bacterium by studying ferredoxins
Small proteins called ferredoxins play a pivotal role in the main metabolic pathways, the series of chemical reactions occurring within a cell.
A team of researchers from Skoltech, MIPT, the Institute of Bioorganic Chemistry of the National Academy of Sciences of Belarus (IBOCH NAS), and the Institute of Biomedical Chemistry of RAS have studied the structures of ferredoxins from the tubercle bacillus and their complexes with partner proteins. The team's findings will help find targets for new anti-tuberculosis drugs. The study came out in Frontiers in Molecular Biosciences.
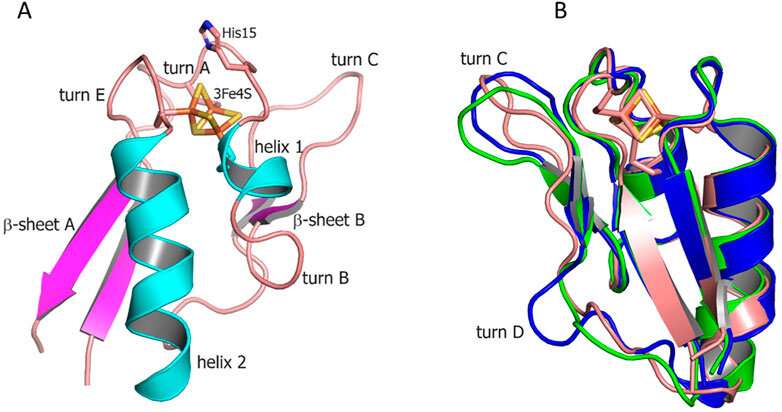
Ferredoxins, which contain an iron-sulfur cluster, are among the most ancient proteins on Earth. They are responsible for carbon dioxide reduction, respiration, and other cellular processes related to electron transfer. Different amino acid compositions and iron-sulfur cluster structures account for the broad diversity of ferredoxins, which perform various functions in human cells and those of other organisms.
Although scientists have discovered and described the genes of many ferredoxins, their protein partners—the molecules they interact with—and the interaction mechanisms still remain obscure for many ferredoxins.
Looking at Mycobacterium tuberculosis, which has five ferredoxins encoded in its genome, the researchers noticed that two 3Fe-4S-ferredoxins are located next to the genes of P450 cytochromes, proteins involved in important intracellular reactions and emerging as potential targets for new anti-tuberculosis drugs. Such gene proximity might indicate a functional relationship between ferredoxins and cytochromes. And in fact, to function properly, cytochromes do need electrons delivered by protein partners, ferredoxins.
Andrei Gilep, a research scientist at IBOCH NAS, comments, "We studied the properties of two tuberculous ferredoxins, Fdx and FdxE, and how FdxE binds to CYP143."
The researchers obtained the structures of Fdx, CYP143, and their complex, FdxE-CYP143, using crystallography. Having analyzed the structures of ferredoxins, they identified the elements involved in binding protein partners and calculated the electron transfer path.
The FdxE and CYP143 genes were found to be in close proximity (in the same operon) in the M. tuberculosis genome, which suggests that they function in tandem. To confirm the assumption, the researchers analyzed their specific interactions using the surface plasmon resonance method. The results revealed a high affinity of these proteins, confirming the hypothesis.
Further study of thermodynamic parameters showed that electrostatic interactions and hydrogen bonds dominate the interaction between partners.
Natalia Strushkevich, an assistant professor at Skoltech Bio, explains, "Aware that we were dealing with the mycobacterial ferredoxin-cytochrome pair, we decided to obtain the crystal structure of the complex. The high-resolution structure showed how the proteins interact with each other. We discovered numerous hydrogen bonds and electrostatic contacts that confirm the thermodynamics of the protein complex formation. To assess the extent to which binding to ferredoxin affects the cytochrome, we also obtained the cytochrome structure alone and identified the protein elements affected by the interaction."
Proteins need to be crystallized before studying the atomic structure. Since crystallization might strongly affect the molecule, the researchers had to confirm their crystal-based conclusions under conditions more typical for the functioning of proteins.
The team performed experiments to check how FdxE and CYP143 bind in a solution where proteins exist in their near-native state using the small-angle X-ray scattering method, which helped capture the interaction between molecules. Thus, the researchers showed how ferredoxins and P450 cytochromes interact with each other, using FdxE and CYP143 as an example.
Valentin Borshchevskiy, deputy director of the MIPT Research Center for Molecular Mechanisms of Aging and Age-Related Diseases, concludes, "Our findings shed light on the essential structural aspects of the protein interactions within these complexes during electron transfer. However, these systems are yet to be characterized in more detail."
More information: Andrei Gilep et al, Structural insights into 3Fe–4S ferredoxins diversity in M. tuberculosis highlighted by a first redox complex with P450, Frontiers in Molecular Biosciences (2023). DOI: 10.3389/fmolb.2022.1100032
Provided by Skolkovo Institute of Science and Technology