February 15, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists decipher how non-resistant forms of Staph aureus thwart antibiotics, almost like a superbug
Staphylococcus aureus (S. aureus) has a slew of surprises in its bag of dirty tricks and among the most puzzling has been the ability of non-resistant forms of the bacteria to emerge unscathed, even after exposure to high concentrations of chemical warfare from antibiotics that should be able to kill it.
That uncanny capability of non-resistant forms of the bacteria is a trick that allows these microbes to rebuff drugs almost as handily as their drug resistant counterparts. Possession of drug-surviving capabilities is just one of many conundrums to vex scientists who've been attempting to tease out how S. aureus finds ways to dodge deadly concentrations of antimicrobials.
The capacity of the bacteria to remain impervious to powerful medications, scientists say, underlies why a growing number of patients worldwide can't shake infections with non-resistant strains.
"Staphylococcus aureus can cause infections that are often chronic and difficult to treat, even when the bacteria are not antibiotic resistant," reports Dr. Markus Huemer, lead author of a new study that zeroes in on the biological mechanisms undergirding the ability of S. aureus to thwart antibiotics.
Huemer, an investigator in the Department of Infectious Diseases and Hospital Epidemiology at University Hospital Zurich, worked with an international team of microbiologists to uncover how a complex cascade of chemical activities apparently protects the bacteria from antibiotic assault. The finding may eventually help doctors conquer infections caused by non-resistant, yet complex forms of the bacteria that frequently infiltrate human tissues and the bloodstream.
S. aureus is an infection-causing bacterium and there are dozens of strains capable of infiltrating human tissues and the blood. But S. aureus also mystifyingly colonizes the nasal passages of about 30% of people on the planet as part of their microbiome. The capacity of an otherwise infectious bacterium to colonize without causing infection appears to be controlled by the composition of these individuals' nasal microbiota.
The complexity of S. aureus doesn't end there, because more concerning are the drug-resistant forms of the bacteria, lethal menaces known as MRSA—methicillin resistant S. aureus—as well as VISA, or vancomycin-intermediate S. aureus, and VRSA, vancomycin-resistant S. aureus.
MRSA poses a threat not only in health care settings, but in communities where it's transmitted in gyms, locker rooms, schools and countless other locations where people congregate.
The U.S. Centers for Disease Control and Prevention describes S. aureus as one of the most common bacterial species that colonizes humans, and antibiotic-resistant varieties can encumber patients for weeks to months, confining them to long hospital stays and prolonged treatments. Infections also can prove deadly. Nearly 120,000 S. aureus bloodstream infections and 20,000 associated deaths occur annually in the United States, according to the CDC.
Yet, the inescapable scientific puzzle regarding the bacteria revolves around this simple question: How can S. aureus survive lethal doses of potent antibiotics in a non-resistant state? It's a superpower possessed by strains of the bacteria that aren't even superbugs, and it has taken a global team of scientists to fully explain why this capability has emerged in certain S. aureus strains.
Writing in the journal Science Signaling, Huemer and his colleagues have begun to peel some of the mystery involving S. aureus. In a series of experiments, the researchers have figured out how these non-resistant bacterial colonies emerge unscathed. The research provides an intriguing new look into a remarkable, yet previously secret aspect of life for one of the world's most ubiquitous bacteria.
S. aureus is capable of surviving high concentrations of antibiotics because it has evolved a way to dramatically slow its metabolic activities, persisting in a state of near suspended animation "because most antibiotics act only on metabolically active cells," Huemer asserted.
Over evolutionary time, S. aureus has figured out how to slow its growth, a message sent throughout bacterial colonies by a signaling molecule when humans attack S. aureus with antibiotics. The signaling molecule helps control the slowing of bacterial metabolism and growth, forcing S. aureus into a survival mode by becoming less active and more quiescent. When the threat has passed, another molecule signals the colony to again become metabolically active.
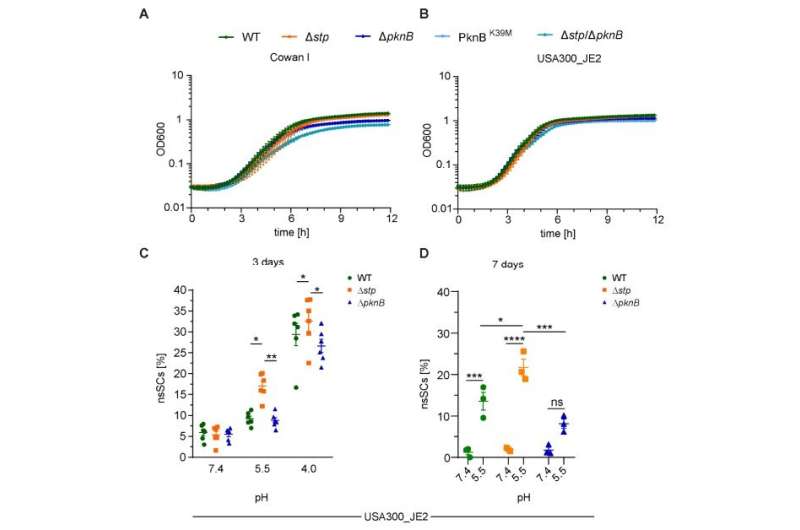
The first of those signaling molecules is called PknB; the second, Stp. Each plays a crucial role helping S. aureus persist despite the chemical warfare being waged against it. Persistence—survival—is so intricately part of the evolutionary history of the bacteria that the bacterial cells clearly display how it's done when scientists put S. aureus under harsh experimental conditions.
"Subpopulations of persister cells are metabolically quiescent, a state associated with delayed growth, reduced protein synthesis, and increased tolerance to antibiotics," Huemer noted, indicating the reduced metabolism allows the bacteria to be totally unfazed by the drugs assaulting it.
As the team knew going into the research, antibiotics have their best kill rates on metabolically active bacteria. S. aureus, however, grinds to a halt, emerging from the antibiotic attack unscathed.
Working with a far-flung team of colleagues in the United States and Australia, Huemer and his collaborators found that these subpopulations of persister cells were made up of non-growing or super-slow-growing S. aureus. These laggards enabled the bacterial colony to survive antibiotic exposure without the resistance mechanisms found in colonies of highly drug resistant forms of the bacteria, such as MRSA.
In their research paper, Huemer and colleagues attribute the persisters' capacity to rebuff antibiotics to the signaling network. As part of their experiments, Huemer and colleagues exposed S. aureus to stressful acidic conditions, similar to those encountered in host tissues.
The acidic conditions delayed the growth of S. aureus, and in so doing, increased bacterial tolerance to various antibiotics. The team also discovered as soon as the antibiotics flood the colony, the PknB molecule is activated.
Chemically, PknB performs a critical task. It signals the addition of phosphate groups to the amino acids serine and threonine. The addition of phosphate groups to these amino acids, helps slow bacterial metabolic activity. When the threat is gone, another molecule, Stp, reverses the activity of PknB, allowing the bacteria to become active again. Huemer and the team used sophisticated tools to check their observations.
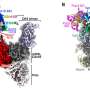
"Using phosphopeptide enrichment and mass spectrometry-based proteomics, we identified targets of serine-threonine phosphorylation that may regulate bacterial growth and metabolism," Huemer wrote in Science Signaling. That means the serine-threonine phosphorylation is a crucial step for S. aureus to bolster itself against antibiotics, even without the highly evolved biological tools drug resistant bacteria use to resist drugs.
The team finalized their report by underscoring that a human treatment strategy can be developed to intervene with the signaling processes by modifying the two mediators—PknB and Stp. They start and stop the serine-threonine phosphorylation, acting as gatekeepers that allow S. aureus to thrive in either a hostile or hospitable chemical environment.
Manipulating them offers a way to control these two pathways, and possibly stop chronic S. aureus infections by eliminating stubborn persister cells.
"Our findings highlight the importance of phosphoregulation in mediating bacterial quiescence and antibiotic tolerance and suggest that targeting PknB or Stp might offer a future therapeutic strategy to prevent persister formation during S. aureus infections," Huemer concluded.
More information: Markus Huemer et al, Serine-threonine phosphoregulation by PknB and Stp contributes to quiescence and antibiotic tolerance in Staphylococcus aureus, Science Signaling (2023). DOI: 10.1126/scisignal.abj8194
Journal information: Science Signaling
© 2023 Science X Network