This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers find a path toward hepatitis E treatment by disentangling its knotty structure
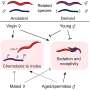
![Structural prediction models suggest conserved cysteines within CxC[x11]CC[x8]CxC motif form divalent ion coordination pockets and novel domain-domain interaction with upstream Y-domain. (a) Alphafold structural predictions of domains within hepatitis E virus (HEV) ORF1. Left: pseudo zinc-finger (amino acids 451–493 within putative papain-like cysteine protease [pPCP]). Magenta: conserved cysteines C457, C459, C471, C472, C481, and C483A. Potential divalent ion coordination tetrahedron outlined in yellow hatched line. Middle: amino acids 242–259 of HEV ORF1 Y-domain, and amino acids 451–462 of HEV pPCP. Conserved cysteines C457 and C459 are outlined in magenta. D248 and H249 of upstream Y-domain highlighted in blue. Novel interdomain divalent ion coordination domain outlined in yellow hatched line. Right: HEV ORF1 protein demonstrating folding of WT and point mutant proteins. Orange (AAs 1–1036): methyltransferase (Magden et al., 2001). Yellow (AAs 1018–1262): Helicase (Devhare et al., 2014; Karpe and Lole, 2010). Red (AAs 1257–1709): RNA dependent RNA polymerase (Koonin et al., 1992; Oechslin et al., 2022). Cyan: Putative membrane association domain (Parvez, 2017). (b) Multiple sequence alignment of HEV genotypes 1–8 of partial Y-domain containing variable residue D248 and highly conserved residue H249. (*) identical residue. (:) similar residue. (.) dissimilar residue. Yellow hatched line – bonds between coordinating amino acids. Credit: eLife (2023). DOI: 10.7554/eLife.80529 Researchers find a path toward Hep E treatment by disentangling its knotty structure](https://scx1.b-cdn.net/csz/news/800a/2023/researchers-find-a-pat.jpg)
In a paper published Feb. 28 in the journal eLife, a team of researchers headed by Princeton's Alexander Ploss settle a debate about a key protein in hepatitis E (Hep E), which could open the way to developing treatments for a tiny virus that poses an outsized threat to public health around the globe.
In their paper, the researchers present a new model that details the structure and function of a Hep E protein.
"Hepatitis E is a poorly understood RNA virus that is responsible for about 3 million symptomatic infections and around 70,000 deaths per year," said Ploss, a professor in Princeton's Department of Molecular Biology.
Hep E is typically transmitted through fecal contamination of water, food or surfaces, so viral disease is more common in regions with poor sanitation. However, outbreaks also arise in places with good sanitation when people eat contaminated foods such as camel or pork. Those infected may suffer fever, nausea and jaundice; and although most recover within two to eight weeks, infection can also become chronic. In some, it is fatal.
"Regrettably, many fatalities occur in pregnant women and their unborn children in late stages of pregnancy, and among the immunocompromised," said Ploss.
Currently, only China has licensed a vaccine to prevent Hep E infection, and there are no drugs available to treat the disease once infection is established. The damage caused by Hep E is all the more remarkable because the virus is so very small; its genome is about 7,200 nucleotides in length and contains instructions to make only three proteins.
"The structure and function of the largest hepatitis E protein, referred to as open reading frame 1—ORF1—is poorly understood," said Ploss.
ORF1 is a multifunctional protein whose job is to make copies of the virus's genetic material for incorporation into new virions. Along its length, it contains several distinct regions that each serve different functions. Many of these regions have already been characterized, but ORF1's size and complexity have made the protein so difficult to study that, until now, researchers still didn't understand how one region of it works.
"Our work aimed at deciphering how a particular region of ORF1 functions, as there is currently a debate in the field on this topic," said Robert LeDesma, a Ph.D. graduate and the first author on the study, who performed the research as a graduate student in Ploss's lab.
The debate centers around the idea that this part of ORF1 might work as a protease (that is, a protein that cuts other proteins). Many viruses encode a protease in their genomes, either to process viral proteins into their active form or else to shut down host proteins that can counteract infection. However, when their initial experiments did not support the idea that this region has protease activity, the Princeton team had to consider other hypotheses.
One striking feature of the area they were studying was the presence of a pattern, or motif, containing eight instances of the amino acid cysteine. This motif appears in every Hep E genome studied so far, which suggests it is quite important to ORF1. Indeed, the Princeton team found that ORF1 can no longer help Hep E replicate if any of the central core of six cysteines is changed to a different amino acid.
Looking for hints as to what this mysterious region might do, the researchers searched protein databases for other proteins that contain the same motif, but whose function is already known. A shorter version of the motif containing only six cysteines is present in proteins that bind a metal ion (such as magnesium or zinc) in order to help stabilize their three-dimensional shape. LeDesma and colleagues reasoned that if the area containing ORF1's cysteine-rich motif has a similar function, then its 3D shape should resemble that of the metal-binding regions in those other proteins.
Other research teams have tried and failed to determine the 3D shape of this part of ORF1 using approaches such as NMR spectroscopy and X-ray crystallography, because this part of the protein is very disordered and tends to assume a variety of random shapes rather than a single rigid one.
Therefore, the researchers instead used a computational algorithm called AlphaFold to predict the region's 3D shape. AlphaFold predicted that ORF1 contains a novel version of a feature common to metal-binding proteins, known as a "zinc-finger," that is needed to interact with metal ions. Subsequent experiments showed that the ability to bind a metal ion is essential for ORF1 to perform its functions in viral replication.
"We discovered that ORF1 behaves like a molecular scaffold; it binds to metal ions within the cell in order to assume a very specific shape that allows it to function properly," said LeDesma.
In other words, the data suggest that this region of ORF1 does not work as a protease, but instead works to structurally support the rest of the protein. With a clearer picture of ORF1, scientists are now in a better position to start attacking it.
"Our work provides a comprehensive model of the structure and function of ORF1 which can conceivably contribute to the development of novel therapeutics for this understudied human viral pathogen," said Ploss.
More information: Robert LeDesma et al, Structural features stabilized by divalent cation coordination within hepatitis E virus ORF1 are critical for viral replication, eLife (2023). DOI: 10.7554/eLife.80529
Journal information: eLife
Provided by Princeton University