This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Heterostructured nanoflowers for high-performance sodium storage
A Chinese team has published new work on battery designs employing heterostructured nanoflowers in Energy Material Advances.
"Sodium-ion batteries (SIBs) have shown to be promising candidate to replace lithium-ion batteries (LIBs), because Na is considered ubiquitous on Earth," said paper author Jun Song Chen, with School of Materials and Energy, University of Electronic Science and Technology of China. "However, the larger atomic size of sodium than lithium results in retarded charge diffusion and severe volume change during sodium storage, thus deteriorating the cycling stability and rate performance of SIBs."
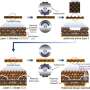
It is crucial to design unique electrode materials with enhanced electrochemical properties to address these drawbacks. Chen explained that transition metal sulfides are attractive for sodium storage among various anode materials. Besides, constructing heterostructures is also effective to enhance the electrochemical properties of active materials, which induces a strong internal electric field at the interface between two components, offering extra driving force for the ions and electrons transport at the boundary.
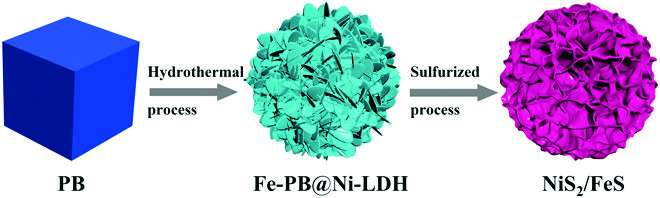
"Inspired by these ideas, we constructed a unique NiS2/FeS heterostructure by a multi-step method," Chen said. "The as-synthesized NiS2/FeS exhibited good sodium storage properties with enhanced high-rate performance of 156 mAh g-1 at 50 A g-1, and high stable long cyclic retention of 606 mAh g-1 at 5 A g-1 after 1000 cycles, much better than other metal sulfide electrodes."
The mechanism of the enhanced performance due to heterostructure need to be confirmed. According to Chen, the density functional theory (DFT) calculations were adopted to uncover the reason for the superior battery performance of the NiS2/FeS heterostructure.
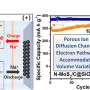
"The NiS2/FeS heterostructure displayed the lowest Na migration energy barrier [compared to] pure NiS2 and FeS, confirming the fastest Na diffusion at the interface. In addition, NiS2/FeS demonstrated the highest adsorption energy, implying that the heterostructure provided the most stable adsorption of Na, thus offered improved structure stability upon repeated charge/discharge. Furthermore, the calculated total DOS (TDOS) showed that the NiS2/FeS heterostructure possessed the maximum charge density near the Fermi level, indicating that the NiS2/FeS possessed enhanced electrical conductivity," Chen said.
"As the DFT calculations showed, the NiS2/FeS heterostructure significantly enhanced the electrical conductivity, kinetic diffusion of Na+ and structure stability, leading to high reversible capacities and superior cycling stability, which matched well with the experimental results."
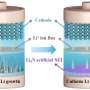
The researchers also explored the potential for commercial applications of as-synthesized NiS2/FeS. "We investigated the full-cell performance by assembling devices using Na3V2(PO4)3 (NVP) as the cathode and NiS2/FeS as the anode," Chen said. "As-assembled NVP||NiS2/FeS full cell exhibited good electrochemical performance, with good commercial potential."
This work provides an effective approach to design composite functional materials with heterostructures for different applications with improved properties.
More information: Dong Yan et al, NiS 2 /FeS Heterostructured Nanoflowers for High-Performance Sodium Storage, Energy Material Advances (2023). DOI: 10.34133/energymatadv.0012
Provided by Beijing Institute of Technology Press Co., Ltd