This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Electrocatalysis: Iron and cobalt oxyhydroxides examined

Very soon, we need to become fossil free, not only in the energy sector, but in industry as well. Hydrocarbons or other raw chemicals can be produced in principle using renewable energy and abundant molecules such as water and carbon dioxide with the help of electrocatalytically active materials. But at the moment, those catalyst materials either consist of expensive and rare materials or lack efficiency.
A team led by Dr. Prashanth W. Menezes (HZB/TU-Berlin) has now gained insights into the chemistry of one of the most active catalysts for the anodic oxygen evolution reaction (OER), which is a key reaction to supply electrons for the hydrogen evolution reaction (HER) in water splitting. The hydrogen can then be processed into further chemical compounds, e.g., hydrocarbons. Additionally, in the direct electrocatalytic carbon dioxide reduction to alcohols or hydrocarbons, the OER also plays a central role.
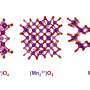
A highly promising class of electrocatalysts for OER are Cobalt-Iron Oxyhydroxides. The scientists analyzed a series of LiFe1-xCox borophosphates at BESSY II with different spectroscopy techniques to determine the oxidation states of the element Iron (Fe) in different configurations.
Iron: Higher oxidation states and shorter bond distances
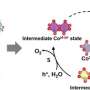
"Fe plays an important role in Co-based OER catalysts. However, the exact reason for this is still under debate. Most studies assume/measure Fe in lower oxidation states (+3) as a part of the active structure. In our case, however, we could show Fe in oxidation states ≥ 4 and shortened bond distances which provide us a better understanding of the catalytically active species," Menezes points out.
Electrocatalysts facilitate the charge transfer from the substrate (here water) to the electrodes, which mostly involves a change of the transition metal oxidation states. However, these oxidation state changes are sometimes too quick to be detected, which makes it hard to understand the working principle of the catalyst especially when it contains two potentially active elements.
This work emphasizes the geometrical structure of the active sites and on the redox behavior of the two participating elements (Co and Fe in the present case). Such an understanding helps to enable design guided development of catalysts on a molecular level. "We hope that the detailed electronic and structural description can substantially contribute to the improvement of OER catalysts," Menezes says.
The work is published in the journal Advanced Energy Materials.
More information: Lukas Reith et al, In Situ Detection of Iron in Oxidation States ≥ IV in Cobalt‐Iron Oxyhydroxide Reconstructed during Oxygen Evolution Reaction, Advanced Energy Materials (2023). DOI: 10.1002/aenm.202203886
Journal information: Advanced Energy Materials
Provided by Helmholtz Association of German Research Centres