This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Developing a weak-acid washing strategy for layered nickel-rich cathodes
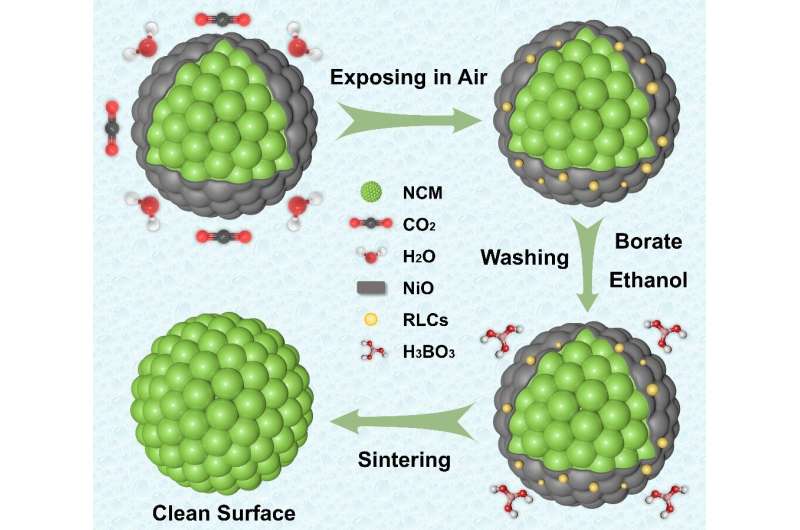
New work on developing a weak-acid washing strategy for layered nickel-rich cathodes was published in Energy Material Advances.
"Satisfying the requirements of cost and performance is imperative for commercialized batteries," said paper author Lai Chen, associate professor at School of Materials Science and Engineering in Beijing Institute of Technology. "Currently, lithium-ion batteries have been widely adopted in the vehicle market, but they still require further development to maintain competitive advantage among competitors."
Chen explained that automotive-type lithium-ion batteries have strict requirements on the cathode materials, such as high specific capacities, suitable discharge potentials, rapid transport kinetics, and good structure stability.
"Among the various candidates of cathode materials, layered transition metal oxides are highly attractive due to their high specific capacities and working voltages," Chen said. "In terms of nickel contents, layered nickel-rich cathode with nickel content above 80% have advantages in capital cost, gravimetric capacity, and energy density, which are important factors for the commercial application."
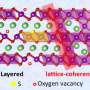
But more severe problems always come along with higher nickel fractions in layered nickel-rich materials. According to Chen, one of the most aggravating problems is the formation of surface impurity phases like the rock-salt NiO phase and residual lithium compounds.
The formation mechanisms of these impurity phases are ascribed to the intrinsic properties of layered nickel-rich materials. The insulating rock-salt phase blocks the Li+/electron diffusion pathways, worsening the electrochemical performance of layered nickel-rich materials. The residual lithium compounds will deteriorate the comprehensive performances and cause trouble in the subsequent electrode manufacturing process.
To remove these harmful surface impurity phases for practical application, Chen said most battery companies have adapted the washing process to stir the cathode powders in purified water for a few hours. These washing processes could significantly reduce the surface impurity phases and powder pH value. However, the increased chemically sensitive and prolonged process time still raise the capital costs. Chen and his team reviewed the latest work, comparing the several modification strategies to eliminate undesired surface impurity phases in which future research should focus.
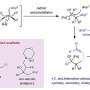
"Notably, the use of acidic treatments has also been demonstrated to eliminate the surface impurity phases and alter the surface structure of Ni-rich materials," Chen said. "In this paper, layered nickel-rich material is etched with a trace amount of boric acid and used as a model to demonstrate the influences of weak acid treatment on the surface phase regulations. Our aim is to provide inspiration for future research in the field."
"The different acid treatment always contains three stages, including the acid-base neutralization between surface impurity phases and acid, Li+/H+ ion exchange and transition metal dissolution reactions between crystal structure and acid, and potential coating reactions during the secondary calcination process."
According to Chen, considering the difference in particle size, inhomogeneity of surface impurity phases, and non-instantaneous characteristics of the etching process, it is hard to precisely control the reaction degree between Ni-rich cathode and acid solution.
"Different from the harmful Li+/H+ exchange and transition metal dissolution reactions during flushing with plenty of water or strong acids, this treatment of ethanol solution with a trace of H3BO3 will mediate the reaction with intrinsic NiO phase and residual lithium to maintain the structure stability and prolong the cycle life," Chen said.
"Our work only uses lower concentrations and short-duration H3BO3 washes to induce chemical changes and improve the electrochemical stabilities of Ni-rich cathode, which shows better storage performance and is of great significance for practical application."
"We provided a novel, efficient, and low-cost strategy to eliminate the surface impurity phases and improve the electrochemical performances of layered nickel-rich material," Chen said. "Such weak acid washing strategy is suitable for large-scale production, thus promoting the potential practical application."
"Our findings confirmed the negative effects of intrinsic NiO phase and residual lithium compounds on the surface of Ni-rich materials and improved the traditional understanding of acid modification," Chen said.
"The effect of washing parameters such as solid-liquid ratio, washing time, and the concentration of weak acid to materials is still an issue to be systematically examined. In addition, further studies are needed to make a real-time verification of these potential chemical reactions between weak acid and surface impurity phases. Overall, there is a long way to go for the large-scale application of weak acid washing process."
More information: Feng Wu et al, Removing the Intrinsic NiO Phase and Residual Lithium for High-Performance Nickel-Rich Materials, Energy Material Advances (2023). DOI: 10.34133/energymatadv.0007
Provided by Beijing Institute of Technology Press