This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How T-shaped clusters drive lanthanide separation during liquid-liquid extraction
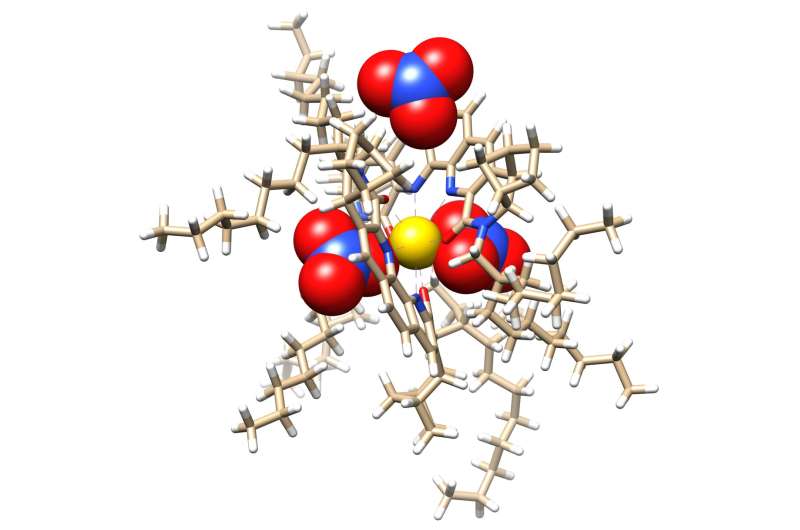
Researchers at Oak Ridge National Laboratory have zoomed in on molecules designed to recover critical materials via liquid-liquid extraction, or LLE—a method used by industry to separate chemically similar elements.
The team had previously designed a novel ligand, or collector molecule, to grab select lanthanides from rare-earth mineral solutions.
Lanthanides are rare-earth metals critical to energy and national security technologies for magnets, electronics and catalysts. They occur together naturally in mineral ore deposits, but their chemical similarities make separating individual elements difficult. LLE methods leverage self-separating liquids such as oil and water to isolate a target material. One example is dividing light and heavy lanthanides. The new study describes how the process unfolds, finding that an unexpected T-shaped cluster forms around target metals, acting like a magnet to create larger aggregates.
"These atomic-scale details are difficult to observe and could help us improve future rare-earth separation strategies," said ORNL's Alex Ivanov.
The research is published in The Journal of Physical Chemistry Letters.
More information: Darren M. Driscoll et al, Noncoordinating Secondary Sphere Ion Modulates Supramolecular Clustering of Lanthanides, The Journal of Physical Chemistry Letters (2022). DOI: 10.1021/acs.jpclett.2c03423
Journal information: Journal of Physical Chemistry Letters
Provided by Oak Ridge National Laboratory