This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
One-pot reaction creates versatile building block for bioactive molecules
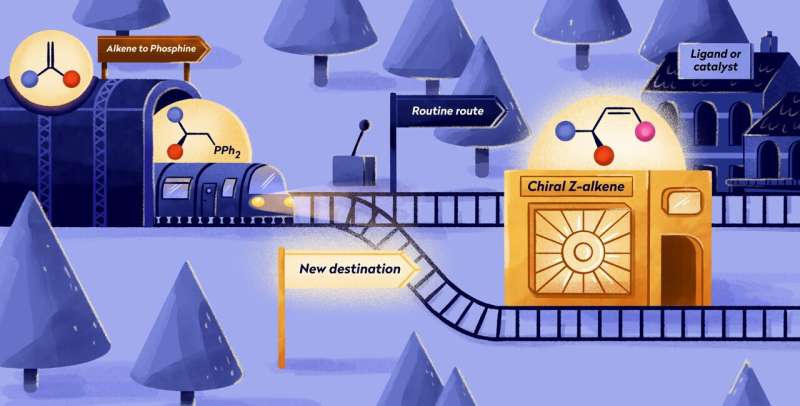
Chemists from the University of Groningen have found a simple way to produce previously inaccessible chiral Z-alkenes, molecules that offer a significant synthetic short-cut for the production of bioactive molecules.
Instead of eight to ten synthetic steps to produce these molecules, the new reaction can be done in three steps, without the need for any purification. The key lies in a phosphine molecule that is normally used to make metal-containing catalysts but that turns out to be the ideal starting point for this chemical reaction. The results were published in Science Advances on January 13.
Organic compounds are very versatile. Their carbon atoms can be connected by single, double, or triple bonds. Furthermore, many biologically important molecules contain chiral centers, parts of the molecule that can be in two mirror image positions, comparable to a left and a right hand. Molecules that have a double bond, a chiral center, and a reactive group for synthetic modifications all next to each other are also important, but chemists find that these are very difficult to make.
Unstable
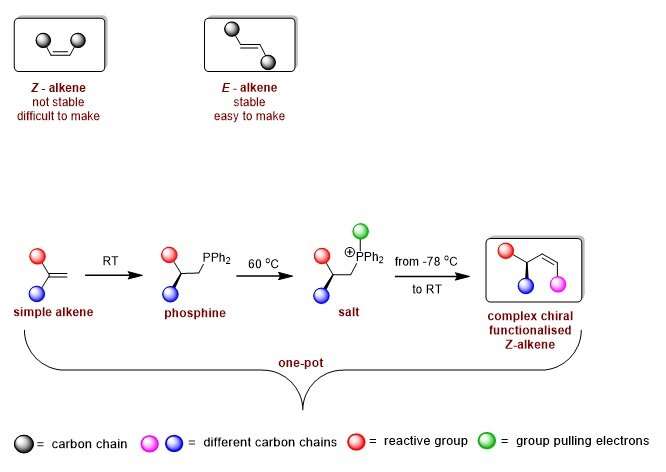
Alkenes are compounds that contain two carbon atoms that are connected by a double bond. When picturing these two carbon atoms horizontally, we can distinguish Z-alkenes, whereby both carbon atoms are connected to another carbon on the same side (both pointing up), and E-alkenes, whereby the connected carbons are on opposite sides, (one up and one down). Z-alkenes are unstable because carbons that are connected on the same side are forced to be close to each other.
"The molecule doesn't like this and if it gets a chance it will transform into the more stable E-alkene. That is why it is hard to make less stable Z-configured alkenes," explains Syuzanna Harutyunyan, Professor of Homogeneous Catalysis at the University of Groningen. "Z-alkenes are very useful, but also difficult to make."
The team needed to make the less stable Z-alkenes, where the double bond is connected to a chiral carbon center, further connect to a highly reactive carbon center, which is very tricky.
Reactive salt
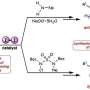
Using known synthetic methods, it would take about eight to ten separate steps to create such a structure. Harutyunyan and her team tried to simplify this by starting out with a molecule called phosphine. Co-author Roxana Postolache says, "This molecule is normally used to produce metal-containing catalysts. In previous work, we developed a way to make a chiral phosphine, which formed the basis for our new synthetic route to Z-alkenes."
Harutyunyan states, "We took our phosphine and turned it into a salt. This would allow the creation of a double bond with Z-configuration."
But this salt is very reactive and all the attempts to introduce a double bond resulted in a lot of products that the scientists did not want. "So, we had to find a way to tune the reactivity," explains Postolache.
Blackboard
This step required a blackboard and chalk approach, which Harutyunyan and her team used to discuss options. A potential solution was found in adding a special group to the phosphine to make a different type of salt. Harutyunyan says, "We figured that this should pull electrons away from the phosphorus and would allow us to tune the reactivity."
First author Luo Ge took the idea from the blackboard to the laboratory. "We tried to make this idea work and we got it right with our first attempt. It was a pleasant surprise to see that our idea really worked." They subsequently optimized the reaction and then used their method to modify real bioactive compounds.
Possibilities
A big advantage of the new synthetic route is that it takes fewer steps and is essentially a one-pot reaction. It just requires room temperature for the first step, mild heating (50–70 °C) to make the salt, and –78 °C for the final step of making the double bond with a Z-configuration.
Joint first author Esther Sinnema says, "By using our phosphine as a synthetic tool, rather than a catalyst, we opened up all kinds of possibilities. We could make a large number of new chiral Z-alkenes and use the method to modify bioactive compounds. In the paper, we present 35 different molecules that were made with our method."
"We expect that our study will pave the way for using commercially available, simple alkenes to make much more complex functionalized alkenes via phosphine and salt intermediates," says Harutyunyan.
More information: Luo Ge et al, Enantio- and Z-selective synthesis of functionalized alkenes bearing tertiary allylic stereogenic center, Science Advances (2023). DOI: 10.1126/sciadv.adf8742. www.science.org/doi/10.1126/sciadv.adf8742
Journal information: Science Advances
Provided by University of Groningen