Successful hypothermia in nonhuman primate paves the way for future application in human torpor during spaceflight
Hibernation is a state adopted by certain mammals as an adaptation to adverse winter conditions. Typical features of hibernation include greatly reduced metabolic activity and lowered body temperature.
As warm-blooded animals, primates (except lemurs) do not naturally hibernate or even experience torpor. But can we manipulate the body temperature of primates and make them fall into a hypometabolic state or even artificial hibernation?
A research team led by Dr. Wang Hong and Dr. Dai Ji from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences has recently reported the first reliable hypothermia in nonhuman primates caused by activating a group of hypothalamic neurons.
The study was published in The Innovation.
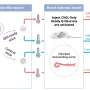
The researchers explored thermoregulation in the nonhuman primate Macaca fascicularis by combining chemogenetic manipulation, functional magnetic resonance imaging (fMRI) scanning, behavioral analysis, and monitoring of a comprehensive set of physiological and biochemical parameters.
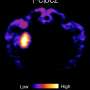
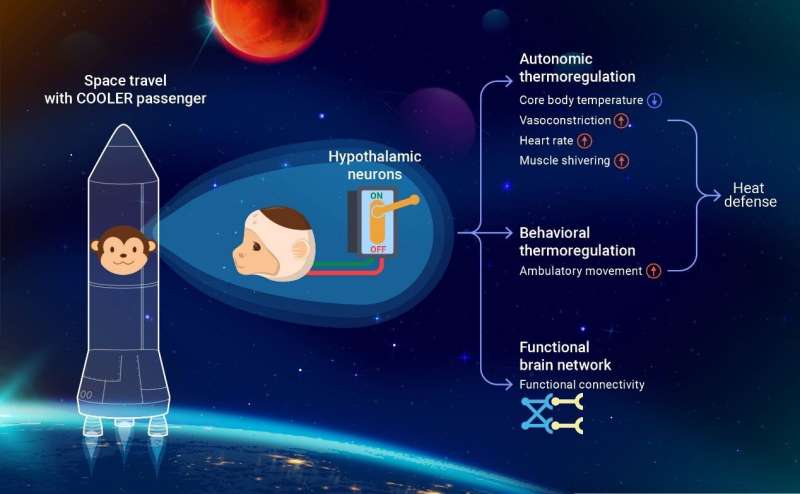
"To investigate the brain-wide network as a consequence of preoptic area (POA) activation, we performed fMRI scans and identified multiple regions involved in thermoregulation and interoception," said Dr. Dai, one of the corresponding authors. "This is the first fMRI study to investigate the brain-wide functional connections revealed by chemogenetic activation."
The researchers selectively targeted excitatory neurons in the POA of the hypothalamus in the monkey brain by locally infecting neurons with DREADD-encoding viruses driven by the CAMKII promoter. "DREADD" refers to designer receptors exclusively activated by designer drugs. They found that activation of the subset of POA neurons by the cognate DREADD agonist Clozapine N-oxide (CNO) reliably triggered hypothermia in anesthetized and awake monkeys.
In the anesthetized experiments, surprisingly, CNO-induced neuronal activity induced a decrease in core body temperature, antagonizing external heating. This demonstrates that the evolutionarily conserved excitatory neurons in the POA are functionally conserved as well and play a critical role in thermoregulation in the primate brain.
The researchers examined the autonomic and behavioral responses to the induced hypothermia in the monkey model. In contrast with mice, which typically decrease activity and lower heart rate, monkeys defend their body temperature by a boost in heart rate, shivering of their skeletal muscles, and an increase in locomotion. All the data point to the notion that primates' thermoregulation mechanism is more complex than in mice. Anatomically conserved cell-types may diverge in their connections and functions.
"This work provides the first successful demonstration of hypothermia in a primate based on targeted neuronal manipulation," said Dr. Wang. "With the growing passion for human spaceflight, this hypothermic monkey model is a milestone on the long path toward artificial hibernation."
More information: Zhiting Zhang et al, Primate preoptic neurons drive hypothermia and cold defense, The Innovation (2022). DOI: 10.1016/j.xinn.2022.100358
Provided by Chinese Academy of Sciences