Researchers investigate the role of sulfides in aerobic/anaerobic switching in bacteria
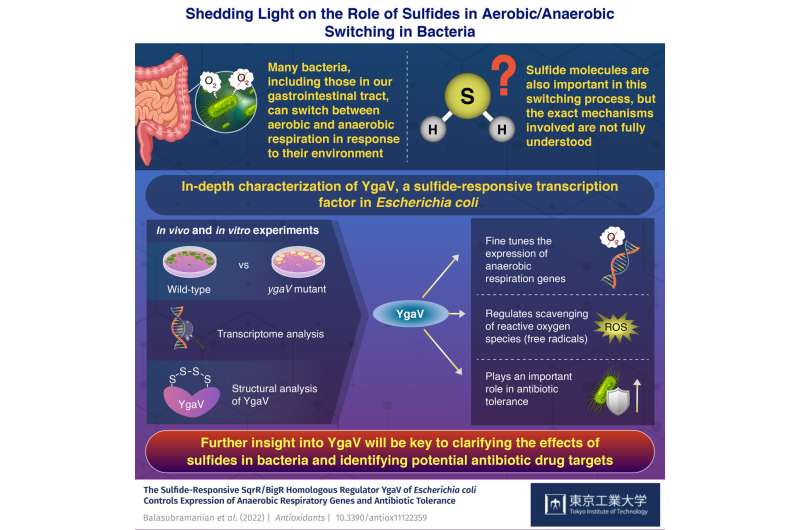
The bacteria in our intestinal tract play many essential roles in maintaining our health and metabolism. Keeping these organisms happy and striking a good balance among species is therefore very important to remaining healthy. Many of these bacteria have special mechanisms by which they can switch from aerobic respiration (using oxygen) to anaerobic respiration (without using oxygen) depending on their environment.
This peculiar switching process is also involved in other functions, such as increasing antibiotic tolerance and protecting the cell against reactive oxygen species (ROS), a family of potentially harmful free radicals. Interestingly, small sulfur-containing molecules called sulfides also affect this switching process. Bacteria seem to have evolved ways to sense hydrogen sulfide (H2S) levels and adapt their metabolism accordingly. Exactly how they do this, however, is not well understood.
To address this knowledge gap, a research team including Associate Professor Shinji Masuda from Tokyo Institute of Technology, Japan, recently focused on YgaV, a transcription factor found in the enteric bacterium Escherichia coli, that could add some pieces to the puzzle.
In their latest paper, which was published in Antioxidants, the team explains that YgaV is evolutionarily related (and almost identical) to certain proteins previously discovered in other bacteria that respond to H2S levels. This motivated them to decipher the role of YgaV and how it helps E. coli thrive when the chemical conditions around them change.
The researchers conducted a series of experiments in normal (or WT, for "wild-type") and mutant E. coli strains, the latter of which had a non-functional YgaV gene. They exposed these cultures to different atmospheric conditions (oxygen-rich, oxygen-deficient, and H2S-rich). Then, through transcriptome analysis, the team investigated which genes were expressed differently between the WT and mutant strains, thus highlighting the role of YgaV.
They also exposed both strains of bacteria to several types of antibiotics and compared their effects. Further, they measured the cellular concentration of oxygen peroxide (H2O2), a type of ROS, and analyzed some of the structural details of how YgaV binds to sulfur atoms.
Overall, the results showed that YgaV is crucial for fine-tuning the expression of various anaerobic respiratory genes and managing the levels of ROS in response to external H2S. Notably, the antibiotic tolerance was different between strains, as Associate Professor Masuda remarks: "While the WT bacteria showed increased antibiotic tolerance under H2S-atmospheric conditions, the YgaV mutant did not. Additionally, antibiotic sensitivity was higher in the mutant than in the WT strain, both in the presence and absence of external H2S."
These findings clarify some of the mechanisms by which various bacteria can regulate their internal chemical balance in response to changes in their surroundings. This knowledge could be used to identify potential drug targets to make pathogenic bacteria more susceptible to antibiotics. In turn, this would help in the treatment of infectious diseases. Most importantly, the link that exists between YgaV, antibiotic tolerance, and ROS levels can be studied in more detail to gain a better understanding of how antibiotic resistance works.
"Further research on YgaV may contribute to solving the decade-old enigma of whether there is a relationship between ROS generation and antibiotic tolerance," notes Masuda.
More information: Rajalakshmi Balasubramanian et al, The Sulfide-Responsive SqrR/BigR Homologous Regulator YgaV of Escherichia coli Controls Expression of Anaerobic Respiratory Genes and Antibiotic Tolerance, Antioxidants (2022). DOI: 10.3390/antiox11122359
Provided by Tokyo Institute of Technology