Exploring formation of oligomers from reactions of hydroperoxide esters with Criegee intermediates
Hydroperoxide esters, formed in the reactions of carbonyl oxides (also called Criegee intermediates, CIs) with formic acid, play a crucial role in the formation of secondary organic aerosol (SOA) in the atmosphere.
Recently, a research group from the Institute of Earth Environment of the Chinese Academy of Sciences (IEECAS) investigated the oligomerization reaction mechanisms and kinetics of distinct CIs (CH2OO, syn-CH3CHOO, anti-CH3CHOO and (CH3)2COO) reactions with their respective hydroperoxide esters.
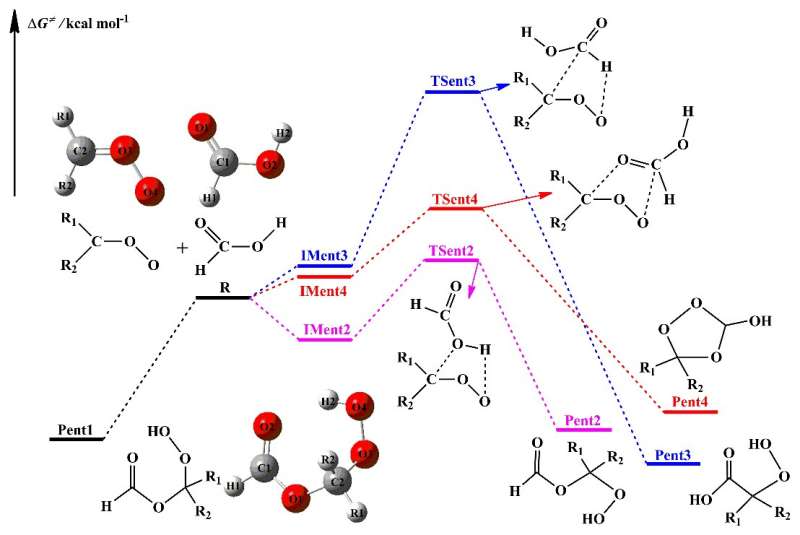
They found that the barrierless 1,4 O-H insertion reaction leading to the formation of hydroperoxide esters was the most favorable pathway in the initiation reactions of distinct stabilized Criegee intermediates (SCIs) with HCOOH. The exothermicity of distinct CIs reactions with HCOOH decreased when the number of methyl groups increased, and the exothermicity of the anti-CH3CHOO + HCOOH reaction was higher than that of the syn-CH3CHOO + HCOOH system.
The addition reactions of CIs with hydroperoxide esters proceeded through successive insertion of CIs into hydroperoxide ester to form oligomers that involve SCIs as the repeating unit. These oligomerization reactions are strongly exothermic and spontaneous.
The saturated vapor pressure and saturated concentration of the adduct products formed from the successive reactions of SCIs with HCOOH decreased significantly as the number of SCIs was increased.
The research is published in the journal Atmospheric Chemistry and Physics.
More information: Long Chen et al, Oligomer formation from the gas-phase reactions of Criegee intermediates with hydroperoxide esters: mechanism and kinetics, Atmospheric Chemistry and Physics (2022). DOI: 10.5194/acp-22-14529-2022
Journal information: Atmospheric Chemistry and Physics
Provided by Chinese Academy of Sciences