Chemists develop reactions for the general synthesis of promising unexplored compounds
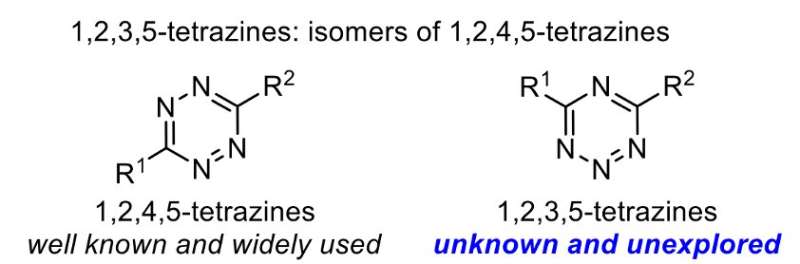
Chemists at Scripps Research have devised the first general method for synthesizing a family of compounds called 1,2,3,5-tetrazines, which hold great promise for making pharmaceuticals, biological probes and other chemical products.
The researchers had synthesized the first compound in this previously unknown family in 2019, but the new method, reported on December 3, 2022 in the Journal of Organic Chemistry, is now general and more efficient.
"For the first time, the chemistry community can access these promising compounds and explore their interesting properties," says study senior author Dale Boger, Ph.D., the Richard and Alice Cramer Professor of Chemistry at Scripps Research.
The study's first author was Zhi-Chen Wu, Ph.D., a graduate student in the Boger lab during the study, now a medicinal chemist at Amgen.
Methods enabling the synthesis of new compounds always offer the prospect of novel medicines and other products with unusual, valuable features. The 1,2,3,5-tetrazines have been seen as particularly promising, given the success of closely related 1,2,4,5-tetrazines. The latter compounds, discovered in 1959, have unique patterns of reactivity and are widely used for making pharmaceuticals, new materials and chemical probes that label biological molecules. The 1,24,5-tetrazines are best known for their uses in "click chemistry" reactions, so-called for their ease of use and efficient, focused reactivity with target molecules.
"The 1,2,4,5-tetrazines have become unbelievably valuable for chemistry in the 60+ years since their discovery," Boger says.
Despite being an isomer of 1,2,4,5-tetrazines—meaning having the same chemical formula, but with a different arrangement of atoms—1,2,3,5-tetrazines have been much more elusive. Yet when Wu and Boger achieved the first 1,2,3,5-tetrazine synthesis in 2019, they found ample evidence of its promise. One observation was that the compound can very efficiently and swiftly react with compounds called amidines via "ligation reactions" (a type of reaction that joins two fragments together). The chemists noted that such ligations could be the basis for new molecular probes and labeling techniques for biology, as well as for assembling pharmaceuticals and other chemical products. The researchers also found evidence that the 1,2,3,5-tetrazine's reactivity differed from that of 1,2,4,5-tetrazines, in a way that could allow the two tetrazine classes to be used simultaneously in certain contexts without generating crossover reactivity.
That first synthesis of a 1,2,3,5-tetrazine was relatively laborious and could be used for making only the one compound. The new method, by contrast, offers a general route to making myriad versions of these compounds—efficiently, in just five reaction steps from inexpensive commercially available starting compounds.
The researchers and their colleagues at Scripps Research will now be synthesizing additional new 1,2,3,5-tetrazines to explore their properties in click chemistry and other applications.
"I think this is the start of a new chapter that could prove to be as timely, durable, and important as the one for 1,2,4,5-tetrazines," Boger says.
More information: Zhi-Chen Wu et al, 1,2,3,5-Tetrazines: A General Synthesis, Cycloaddition Scope, and Fundamental Reactivity Patterns, The Journal of Organic Chemistry (2022). DOI: 10.1021/acs.joc.2c02687
Provided by The Scripps Research Institute