Chemists create framework for the oxidation of hydrocarbons
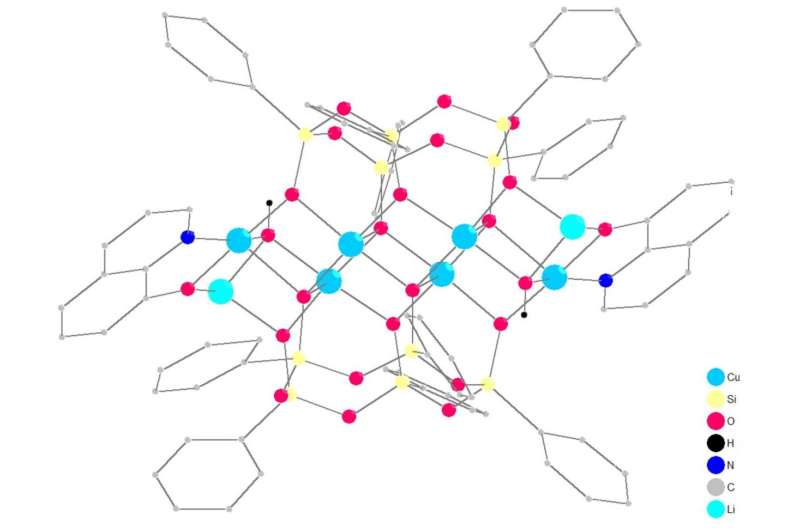
RUDN University chemists have created new copper-containing metallasilsesquioxane frameworks. Some of them have proven to be effective catalysts for the oxidation of hydrocarbons. The results are published in Molecules.
Silsesquioxanes are a large group of organosilicon compounds. They are composed of a silicon and oxygen backbone, with the silicon atom containing an organic substituent. Silsesquioxanes can bind metals to play the role of ligands, and metal-containing derivatives of silsesquioxanes are used in industrial chemistry for catalysis. In particular, complexes of silsesquioxanes with metals catalyze the oxidation of hydrocarbons.
In the new study, a RUDN University chemist created a series of new silsesquioxane complexes with copper and additional organic ligands, 8-hydroxyquinolines. Some of them proved to be effective catalysts.
"Silsesquioxane compounds are a kind of bridge between organics and inorganics. They consist of a purely inorganic main chain of silicon and oxygen; substituents at the silicon atom are responsible for the organic component. Silsesquioxanes are often considered as unique molecular models for surface studies and for catalysis. Structural features of silsesquioxanes are used to create organic-inorganic materials. Another important feature of them is the role of ligands for metal complexes," said Alexey Bilyachenko, Doctor of Sciences in Chemistry, Leading Researcher of the Joint Research Institute of Chemistry, RUDN University.
"At the same time, in the composition of metal complexes silsesquioxanes can combine with additional organic ligands. Surprisingly, the ligands of the very popular 8-hydroxyquinoline family have never been used to design metallosilsesquioxane complexes. We investigated the synthesis of the first such compounds, studied their molecular and supramolecular structures, and also evaluated the properties of new compounds in the oxidation of hydrocarbons."
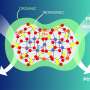
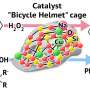
Chemists synthesized a family of 11 compounds. All of them have a similar structure, a three-dimensional "skeleton" with two silsesquioxane and two 8-hydroxyquinoline ligands, coordinating copper and alkali metal atoms (lithium, sodium or potassium). The synthesis goes by self-assembly flow, when simple reagents are transformed into a complex structure. RUDN University chemists described in detail the structure of new compounds, and then tested their catalytic abilities using the example of hydrocarbon oxidation.
One of the obtained compounds turned out to be an effective catalyst in the oxidation of hydrocarbons and alcohols using hydrogen peroxide. Chemists concluded that the key mechanism in oxidation is radical, involving hydroxyl fragments.
"We showed the first examples of wireframe metallosilsesquioxanes containing 8-hydroxyquinoline ligands using a convenient self-assembly method. One of the frameworks showed high catalytic activity in the oxidation of hydrocarbons and alcohols. We came to the conclusion that oxidation proceeds with the participation of free hydroxyl radicals," said Alexey Bilyachenko, Doctor of Sciences in Chemistry, Leading Researcher of the Joint Research Institute of Chemistry, RUDN University.
More information: Alexey N. Bilyachenko et al, A Novel Family of Cage-like (CuLi, CuNa, CuK)-phenylsilsesquioxane Complexes with 8-Hydroxyquinoline Ligands: Synthesis, Structure, and Catalytic Activity, Molecules (2022). DOI: 10.3390/molecules27196205
Provided by Russian Foundation for Basic Research