Sunlight-absorbing organic compounds are produced on the wet surfaces of atmospheric particles
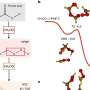
Heterogenous oxidation reactions can occur upon mixing chemicals that are in two different physical states, such a liquid and a gas; for example, in the atmosphere the reaction of gaseous nitrate (NO3) free radicals reaching the wet surface of aerosol particles containing aromatic pollutants from wildfires.
In such a situation, the heterogeneous reaction converts phenols into sunlight-absorbing nitroaromatic compounds. The process should be important during nighttime, when the concentration of nitrate radical peaks and hazardous contaminants can be transformed on the surface of suspended particles by mechanisms not previously considered by atmospheric scientists.
Given the vast distribution of particles found in air, this nighttime process should be quite frequent in regions impacted by pollution from wildfires or from fossil fuel combustion. Therefore, phenolic molecules can play an active role in changing the absorption of sunlight by atmospheric particles and the chemical structure, which in turn affect air quality and climate.
"Previous textbook material generally explained that nitroaromatic compounds are formed by gas phase reactions of nitration, but our new work demonstrates that reactions on wet aerosol surfaces are highly effective to produce nitrophenols," said Prof. Marcelo Guzman from the Department of Chemistry at the University of Kentucky.
"There is no previous scientific consideration of nitration reactions occurring at the interface of water and air, the way that such processes are initiated, or the mechanisms by which nitrate radicals can contribute to such reactions."
Phenols are primary pollutants released to air during wildfires or produced in the atmosphere when solvents from fossil fuel refineries and other industries leak to air and are oxidized. The study, published in Environmental Science & Technology, reports that phenols favorably transfer an electron to nitrate radicals, or temporally attach to the molecule.
These mechanisms should work for other atmospheric compounds reacting with nitrate radicals. For example, during the breakup of aromatics exposed to ozone, the molecule of muconic acid is formed. The study also compared the results of the nitration reactions to those driven by ozone at the interface of water and air.
Related studies have shown that the new yellowish molecules produced absorb more sunlight, increasing the absorption properties of atmospheric particles. Based on the larger absorption of sunlight by the products, the term brown carbon is often used to refer to this yellowish material.
Prof. Guzman concluded, "The formation of yellow organic nitrate products increases the absorbance of atmospheric aerosols, likely affecting the radiative forcing of particles, a factor that has been overlooked for a long time."
More information: Md Sohel Rana et al, Oxidation of Catechols at the Air–Water Interface by Nitrate Radicals, Environmental Science & Technology (2022). DOI: 10.1021/acs.est.2c05640
Journal information: Environmental Science & Technology
Provided by University of Kentucky