New research explores how cancer cells spread in human body
For decades, figuring out exactly why cancerous tumors form in the human body has been a goal for scientists, but knowing how cancer cells spread is also key to fighting the often-deadly disease.
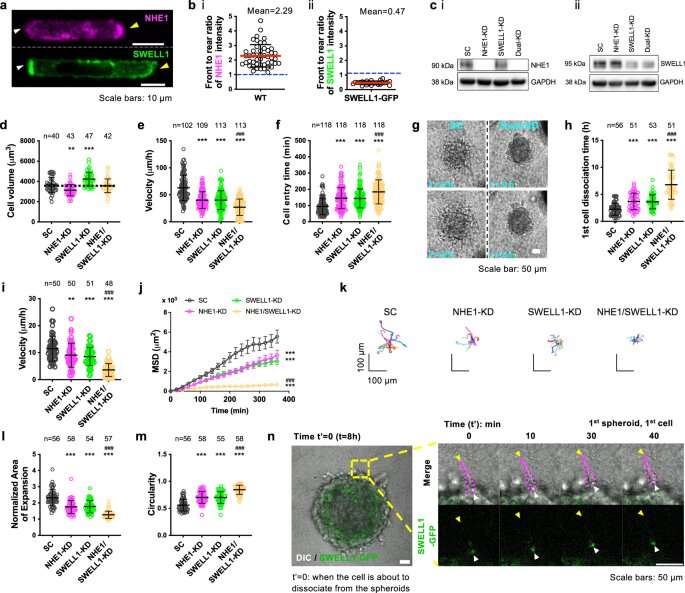
The osmotic engine model of cancer motility has shown that confined cells move by taking in water at the leading edge and expelling it from the back, causing propulsion. However, the exact molecules that regulate those cells' rear shrinkage have remained elusive.
New research published in Nature Communications answers that question about cell locomotion, offering a few more steps along the path to future cancer treatment. Assistant Professor Yizeng Li, who joined the Biomedical Engineering Department at Binghamton University's Thomas J. Watson College of Engineering and Applied Science this fall, co-authored the paper along with collaborators from Johns Hopkins University, the University of Maryland, the University of Alberta and the Universitat Pompeu Fabra in Spain.
Researchers experimented with breast cancer cells in a three-dimensional matrix to study their behavior. As previously confirmed, a molecule called sodium/proton exchanger 1 (NHE1) causes water to be absorbed—but the researchers also discovered that another protein at the back, called SWELL1, polarizes the cell membrane in a way that leads to movement.
"We clearly show that NHE1 concentrated at the front is responsible for intake of water," Li said. "At the back of the cell, SWELL1 will remove chloride—and by removing chloride, it also will remove water. We completed the story about how water goes in and how it leaves."
Li received her Ph.D. from the Department of Mechanical Engineering at the University of Michigan-Ann Arbor, and she was a postdoctoral researcher at Johns Hopkins University's Department of Mechanical Engineering and Institute for NanoBioTechnology. Her background is in theoretical mechanics and applied mathematics with applications to biophysics and mechanobiology.
"Mechanobiology is at the interface of cell biology, physics and mechanics. Most of my work has been concentrated on cell migration, cell volume control and those related questions," she said.
"I developed the mathematical model for this work. To better understand the biophysical mechanisms behind cancer cell motility, I developed a physiology-based model, instead of a phenomenon-based model. The model combines fluid dynamics, cytoskeleton structure and microscopic details such as ionic transportation. The model prediction very much matches the experimental data."
With about 40% of the U.S. population diagnosed with cancer at some point in their lifetimes, research like that from Li and her colleagues could have wide implications for slowing down or halting the deadly disease—even if treatments are years down the road.
"We want to understand under what conditions tumor cells may migrate and under what conditions we can prevent it," she said.
More information: Yuqi Zhang et al, Polarized NHE1 and SWELL1 regulate migration direction, efficiency and metastasis, Nature Communications (2022). DOI: 10.1038/s41467-022-33683-1
Journal information: Nature Communications
Provided by Binghamton University