Researchers have determined the three-dimensional atomic structure of a protein important for organ functions
NKCC1 is a human chloride transporter that has the ability to transport sodium, potassium, and chloride from the exterior into cells. In the kidney, for example, NKCC1 type proteins ensure that these ions are reabsorbed from the urine, and generally NKCC1 is important for osmotic cell volume regulation. In the brain, NKCC1 and related proteins are important for chloride gradients that are vital for the electrical signaling in neuronal networks.
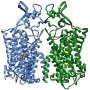
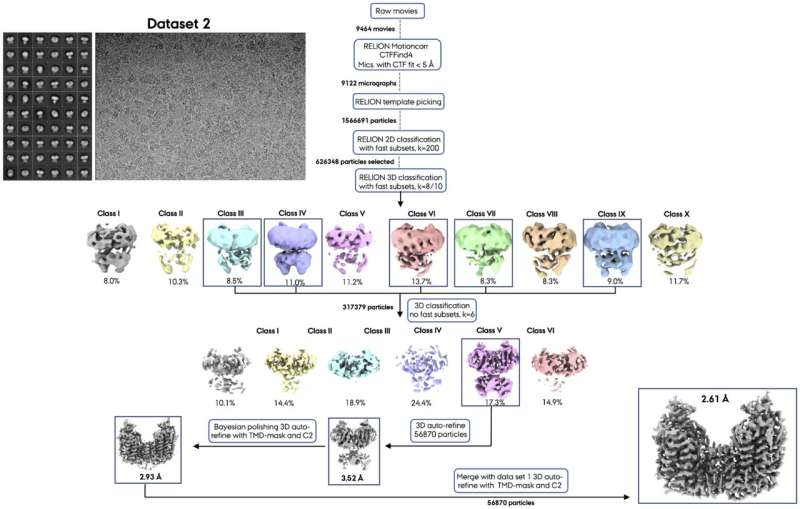
Using cryo-electron microscopy (cryo-EM), a team from Poul Nissen's laboratory with colleagues from the Fenton and Hartmann laboratories at Aarhus University (AU) and the Lindorff-Larsen laboratory at University of Copenhagen (KU) have determined the three-dimensional atomic structure of NKCC1 (a so-called Na+- K+- 2 Cl– cotransporter) and investigated its function.
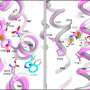
Insights into the three-dimensional atomic structure and dynamics of NKCC1 including the bound ions, lipids and water molecules as well as ion transport studies in cells provide important new information on NKCC1 function, which is driven by the sodium gradient established by the sodium-potassium pump.
The studies that the team present in an article in The EMBO Journal reveal a surprising mechanism for release of the ions into the cell that begins with one of the two bound chloride ions, and only then the sodium ion and finally the other chloride and the potassium ion. The researchers will now continue to try to identify novel compounds that interfere with NKCC1 function and that may help in for example kidney and brain disorders.
More information: Caroline Neumann et al, Cryo‐EM structure of the human NKCC1 transporter reveals mechanisms of ion coupling and specificity, The EMBO Journal (2022). DOI: 10.15252/embj.2021110169
Journal information: EMBO Journal
Provided by Aarhus University