October 17, 2022 feature
A new method to dehydrogenate alkanes at ambient conditions
The chemical term alkanes, or paraffins, refers to organic compounds that consist of single-bonded carbon and hydrogen atoms, such as methane, ethane, and propane, and several other hydrocarbons. Over the years, alkanes have become widely used in organic chemistry, due to their unique chemical properties and their role in producing chemical reactions.
In recent decades, chemists have been exploring the possibility of breaking alkanes down through a process known as "non-oxidative dehydrogenation," to attain valuable carbon feedstocks and hydrogen fuel. This could ultimately have valuable implications for the energy sector, as it would provide a low-cost method of creating clean fuel for new hydrogen-based energy solutions.
Unfortunately, the C-H bonds in alkanes can generally only be broken down at high temperatures, under ultraviolet light, or using stoichiometric oxidants. This hinders the large-scale adoption of this approach to create hydrogen fuel.
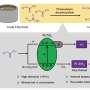
Researchers at Jilin University in China have recently introduced a possible strategy to achieve the non-oxidative dehydrogenation of alkanes in visible to near-infrared light and at room temperature. Their proposed method, introduced in a paper published in Nature Energy, entails the use of platinum/titanium dioxide (Pt/black TiO2) photocatalysts, in which platinum atoms are close but not fully bonded.
"The initial goal of our study was to replace the commercial TiO2 used in my previous work with black TiO2 , to enhance the light-absorption capacity of the catalyst," Lu Li said. "In our experiments, we utilized solar light energy to drive the non-oxidative dehydrogenation of alkanes at room temperature. The introduction of clean photon energy can overcome the thermodynamic limitation, making alkanes conversion occur at ambient conditions with higher selectivity and durability."
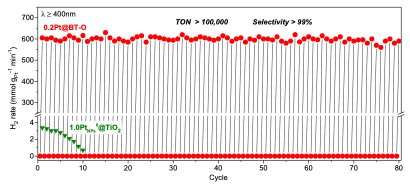
In initial experiments, the approach by Li and his colleagues achieved highly promising results, as it could efficiently dehydrogenate different alkanes at room temperature and in visible to near-infrared light. For cyclohexane, their photocatalysts enabled the production of H2 with a turnover number of 100,000, with the reaction effectively ongoing for 80 reaction cycles. This is a significantly better result than those achieved by thermal reactions.
For methane, on the other hand, the researchers achieved a conversion rate of 8.2%, with a 65% selectivity to propane. Finally, for C2+ alkanes, Li and his colleagues achieved a fast dehydrogenation (up to 1,440 µmol g−1 h−1) to the corresponding olefins.
"The brand-new 'single-atom collection' catalysts can combine the advantages of single-atom catalysts and nano-catalysts," Li said. "This new class of catalysts may have potential applications in many important heterogeneous catalytic processes."
In the future, the photocatalysts used by this team of researchers could prove to be highly valuable for enabling the production of hydrogen fuel from alkanes, without the need for high temperatures, UV-light, and stoichiometric oxidants. This could help to lower the cost of hydrogen fuel production, thus potentially facilitating its use for energy applications.
"To extend this work, we are now looking for efficient photocatalysts based on cheap metals," Li added. "Furthermore, we will extend the substrates to various non-toxic saturated hydrocarbons."
More information: Lili Zhang et al, Visible-light-driven non-oxidative dehydrogenation of alkanes at ambient conditions, Nature Energy (2022). DOI: 10.1038/s41560-022-01127-1
Lu Li et al, Simple and Efficient System for Combined Solar Energy Harvesting and Reversible Hydrogen Storage, Journal of the American Chemical Society (2015). DOI: 10.1021/jacs.5b03505
Journal information: Journal of the American Chemical Society , Nature Energy
© 2022 Science X Network