Capturing extracellular vesicles: A new technology for isolating disease markers
Biophysicists from Skoltech, MIPT and their colleagues from the company Prostagnost have created a new technology for isolating extracellular vesicles (EV) from biological fluids. Studying vesicles is essential for the diagnosis and treatment of various diseases, including cancer. The new technique not only outperforms methods known to date in purity and yield of EVs, but also is simple, fast, inexpensive and can run on standard lab hardware. The research was published in the Journal of Extracellular Vesicles.
Our body cells "communicate" with each other by releasing signaling molecules into the blood flow. For the molecules to safely reach the target, they are encapsulated in tiny, nano-sized vesicles, EVs, that operate as a delivery system. EVs from healthy and diseased cells have different contents, which forms the basis for diagnosis. Vesicles secreted by unhealthy cells contain a whole variety of molecules that serve as a biomarker of a disease. Studying the biomarkers helps both to diagnose a disease and monitor the treatment by analyzing changes in the number of EVs that contain the selected markers.
However, the question arises as to how to isolate these miniscule carriers. Only EVs should be picked out from among the great multitude of molecules in biological fluids in order to identify the protein molecules they contain, which can be biomarkers of a disease or a sign of good health. Depending on which nucleic acids, such as mRNA or DNA, or proteins are found inside or on the surface of an EV, a conclusion is made on the patient's prospects. Therefore, it is important that these studies be performed quickly, efficiently and at low cost.
Vasiliy Chernyshev, lead author and research scientist at the MIPT Laboratory for the Development of Innovative Drugs and Agricultural Biotechnology and the Skoltech BioPhotonics Lab, says that "currently, several generally recognized methods exist for isolating vesicles, but they are either too cumbersome or requiring specialized equipment, such as an ultracentrifuge. Not every clinic can afford this, and besides, this method has a rather low isolation efficiency."
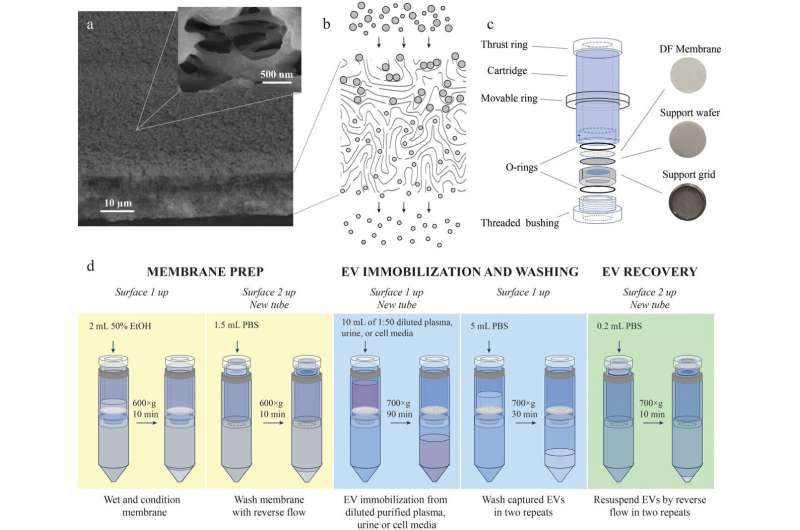
The team has developed a filtration device along with a special membrane composition and design and a step-by-step isolation procedure. The solution enables fast and efficient EV isolation while ensuring high purity, which is very important for both diagnostics and EV research. The device is made entirely from Russian components at minimal cost.
Vasiliy Chernyshev adds that "in the EV isolation device that we developed jointly with the Prostagnost company the separation takes place deep in the membrane with a specific pore design. In contrast to conventional filtration, we capture the product inside the filter and recover it with the reverse flow."
"With this new technique, we can effectively isolate EVs of various sizes, exosomes included, from virtually any biological fluid, such as blood, plasma, and urine, and obtain high-purity EVs free from extracellular particles or molecules. But most importantly, all we need for the task is an ordinary lab centrifuge and specific membranes and test tubes that are accessible for any Russian clinical lab."
Sergey Leonov, Head of the MIPT Laboratory for the Development of Innovative Drugs and Agricultural Biotechnology, comments that "our team has put a lot of effort in describing and proving the purity of the exosomes—membrane vesicles sized 40 to 100 nm. This is highly important for both diagnostics and proteomics."
"There is a great need for such simple, fast and effective methods for scientific and medical EV research. We have proposed a locally developed unique technology that can evolve into a useful routine for conventional oncological practices. This research is a perfect example of MIPT's inter-institutional, industrial and international collaborations that help successfully deal with import substitution tasks and market innovative Russian solutions that largely outperform their international analogs."
More information: Vasiliy S. Chernyshev et al, Asymmetric depth‐filtration: A versatile and scalable method for high‐yield isolation of extracellular vesicles with low contamination, Journal of Extracellular Vesicles (2022). DOI: 10.1002/jev2.12256
Provided by Skolkovo Institute of Science and Technology