How can researchers quickly access complex molecules for drug discovery?
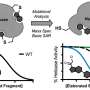
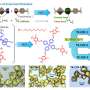
The function of molecules used in drugs in part depends on their structure, including the many chemical bonds between their atoms. These molecules can be built through several different chemical reactions, most of which are slow and inefficient because they rely on the formation of one chemical bond at a time. Ramesh Giri, Weinreb Early Career Professor of Chemistry at Penn State, has developed a reaction that creates two carbon bonds at a time across atoms in a configuration called an alkene with the help of small amounts of nickel, a sustainable and abundant catalyst.
A paper describing the reaction was recently published in the Journal of the American Chemical Society. We talked to Giri about this research:
Q: What comprises the structure of drug molecule?
Giri: Most drug molecules contain many carbon atoms in their structures. A majority of these carbons are connected by carbon-carbon bonds to form a basic framework of the drug molecule, just like the many bones in the human body are connected to form a skeleton. The carbon skeleton of a drug molecule functions as a platform to hold chemical components known as functional groups that impart functional properties to the drug.
Q: What was your motivation for this study?
Giri: The carbon skeletons of drug molecules are created by assembling various carbon sources using reactions that form new bonds between atoms, usually one bond at a time. In many cases, the process of synthesizing drugs therefore becomes protracted and tedious with the involvement of multiple chemical steps with several reaction intermediates, handling of a large number of chemicals, and generation of volumes of chemical wastes. We are developing new, environmentally friendly chemical transformations that are faster, generating multiple bonds in one step and drastically decreasing the number of overall steps.
Q: What were the main results of this study?
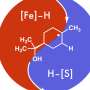
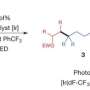
Giri: We have developed a reaction called alkene dialkylation that creates two carbon-carbon bonds across an alkene with the help of nickel, a sustainable and earth-abundant metal, as a catalyst to speed up the reaction. The reaction is incredibly efficient because it is conducted with a much smaller amount of catalyst than usual. We use 500-2000 parts per million (ppm) of nickel compared to 50,000 to 100,000 ppm of catalyst in similar reactions. Our method allows us to synthesize complex molecules rapidly from readily available basic chemicals.
Q: Why is this important?
Giri: There are three important aspects of this new reaction—a) the reaction utilizes a sustainable and Earth-abundant metal as a catalyst, b) the reaction uses the catalyst at extremely low concentrations, making this process the most efficient alkene difunctionalization reaction to date, and c) the new catalytic condition solves one of the most pressing challenges in alkene difunctionalization by adding two functional sites simultaneously. The use of sustainable and Earth-abundant metal as a catalyst will have a broad impact in the synthesis and manufacturing of pharmaceuticals where the cost, availability, and quantity of the catalyst leaves a large footprint on the prices of the pharmaceuticals.
Q: What questions still need to be addressed?
Giri: While the current reaction makes a big stride in this research area, the scope is still limited to two classes of molecules called alkenylarenes and benzyl halides. Although these molecules are among the largest classes of simple and readily available basic chemicals, future work should be focused in expanding the scope to the general class of alkenes, particularly classes called linear unactivated alkenes and alkyl halides.
More information: Roshan K. Dhungana et al. Ni-Catalyzed Regioselective 1,2-Dialkylation of Alkenes Enabled by the Formation of Two C(sp3)–C(sp3) Bonds, Journal of the American Chemical Society (2020). DOI: 10.1021/jacs.0c09778
Journal information: Journal of the American Chemical Society
Provided by Pennsylvania State University